
The therapeutic gold standard for the treatment of severe mitral regurgitation (MR) is elimination of MR by reduction of mitral annular anteroposterior (AP) diameter with direct surgical annuloplasty. However, open heart surgery is declined in many high-risk heart failure (HF) patients who are still symptomatic from MR despite medication.
During the last decade, transcatheter mitral valve repair (TMVr) approaches have earned merit in terms of reduction of MR. Despite excellent echocardiographic and clinical results after MitraClip® (Abbott Vascular, Santa Clara, CA, USA) or PASCAL (Edwards Lifesciences, Irvine, CA, USA) procedures, edge-to-edge TMVr has limitations with about 10-20% functional MR (FMR) >2+ recurrence after 12 months in patients with challenging anatomies1 (and unpublished own data). Moreover, some patients are not suitable for the leaflet repair approach due to large coaptation defects and/or insufficient coaptation length. Furthermore, inadequate leaflet elongation, concomitant leaflet degeneration, especially shortening and unacceptably high transmitral gradients with a single MitraClip or PASCAL, prevent treatment success2. In addition, complete elimination of FMR by transcatheter mitral valve replacement (TMVR) devices still appears to carry higher procedural risk compared to transcatheter repair devices. Consequently, there is a demand for new, minimally invasive transcatheter methods to correct FMR, especially if the patients are ineligible for edge-to-edge repair devices.
Alternatively, direct and indirect transcatheter annuloplasty devices imitating the surgical shortening of AP dimensions address some of the aforementioned limitations. The ARTO™ (MVRx, Inc., Belmont, CA, USA) procedure, a rather indirect annuloplasty, proved to be efficient and safe in a population of high-risk systolic HF patients with severe FMR due to dilatation of AP annulus diameter. The movement of the mitral annulus in the ARTO procedure occurs by distraction of tissue on the level of the left atrium due to the permanent placement of a transatrial horizontal suture. Stretching from one T-bar anchor within the great cardiac vein to an intra-atrial septal (IAS) anchor, its contracting force is transmitted to annular tissue by cinching the IAS and posterior annulus approximately 20-30 degrees posterior to the true AP orientation (Figure 1). Thereby, the ARTO system achieved a 14% reduction in AP diameter at 30 days implanted in 11 high-risk patients (MAVERIC trial, published in 2015) with heart failure with reduced ejection fraction (HFrEF) and FMR3.
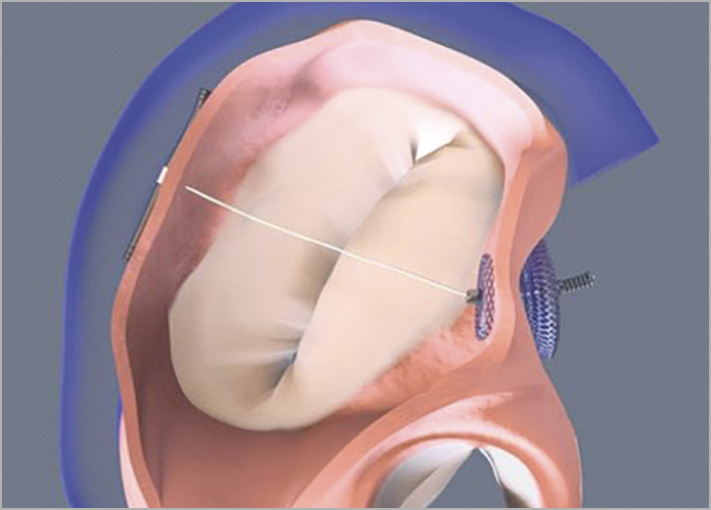
Figure 1. The ARTO device in situ demonstrating decrease in the anteroposterior diameter of the mitral annulus4.
In this issue of EuroIntervention, Worthley et al4] present one-year safety and efficacy results of the transcatheter ARTO system implanted in forty-five patients with FMR, thereby extending the initial results of the first-in-human MAVERIC (Mitral Valve Repair Clinical) trial.
The prospective data originate from a multicentre non-randomised experience in 45 patients with moderate-to-severe or severe FMR – median AP annulus diameter 41.4 mm, effective regurgitant orifice area (EROA) 26.9 mm2 – who were followed for one year. Procedural technical success was high (45/45; 100%) with annulus diameter reduction from 41.4 mm to 35.3 mm achieving a 14.7% reduction in AP diameter at one-year follow-up (p<0.0001). MR grade reduction measured by EROA was 35% from 26.9 mm2 at baseline to 17.6 mm2 at one-year follow-up. Periprocedural complications were reported in seven patients (15.5%), including three minor vascular access-site complications, two major and two minor bleeding events none of which was judged to be device-related. Major adverse events during the follow-up period were rare, with 1 cardiac effusion/tamponade and 1 renal failure at 30 days, 3 and 5 deaths of cardiovascular cause at six months and one year, respectively. Moreover, 1 myocardial infarction, 1 stroke and 1 mitral surgery/intervention occurred up to six months and 2 more renal failures at one-year follow-up. The device success rate was 71.4%, median length of procedure 88 minutes (interquartile range of 65-110 minutes) and median time to discharge was 2 days (interquartile range of 1-5 days), suggesting that the therapy is comparable to other established percutaneous mitral repair therapies.
The ARTO system showed significant echocardiographic and functional improvements. Imaging assessments revealed significant reduction in cardiac volumes with a 14% decrease in indexed left ventricular end-diastolic volume (LVEDVi) (n=35) and 21% of indexed left atrial volume (LAVi) and left atrial volume (n=32). There was a significant improvement in New York Heart Association (NYHA) functional class (n=36) at 30 days which remained significant at one year. Furthermore, the six-minute walk test (6MWT; n=33) and EQ-5D visual analogue health status score (n=26) improved significantly.
With a one-year outcome of 89% survival and 88% without any HF hospitalisations, the MAVERIC trial demonstrated that the ARTO system is a viable concept for the treatment of FMR in HFrEF patients. Similar outcomes were obtained with other currently available alternatives of indirect/direct annuloplasty. With the Cardioband system (Edwards Lifesciences), 87% one-year survival, 66% freedom from HF hospitalisation and 78% freedom from secondary intervention were observed5. The randomised REDUCE FMR trial using the Carillon Mitral Contour System® (Cardiac Dimensions Inc., Kirkland, WA, USA) showed a one-year mortality of 14%, and HF hospitalisations of 11% with 9% having device removal for coronary artery compromise6. In the ARTO procedure, compression or compromise of the left circumflex or coronary artery did not occur.
However, for successful positioning within the market of transcatheter mitral repair devices, three major aspects are of future importance to identify patients who will benefit most from the ARTO procedure:
1. Concise characterisation of criteria on mitral valve anatomies and appropriate patient selection addressing the following parameters: aetiology of HF (e.g., HFrEF only or heart failure with preserved ejection fraction [HFpEF] with left atrial enlargement and consecutive FMR as well), aetiology of MR (FMR only or degenerative MR as well), left atrial dimensions, mitral valve area, coaptation gap, width of the MR jet, low mobility or short leaflet length, and transmitral gradient.
2. Larger randomised controlled clinical trials of patients treated with the ARTO procedure with longer follow-up are needed, analysing endpoints such as mortality, CV mortality and HF rehospitalisation.
3. Simplified guidance for preprocedural simulation and intraprocedural identification of the optimal puncture point of the IAS is a pivotal aspect to gain optimal re-shaping of the mitral annulus.
This will further push the boundaries of TMVr and reduce left ventricular adverse remodelling and dysfunction associated with significant morbidity in HF patients.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

