Device description
Name and manufacturer: Arto™ System; MVRx, Inc., Belmont, CA, USA.
Approval status: Clinical evaluation.
Platform: Left atrial-based mitral valve repair.
Specific design: Anterior-posterior (A-P) annulus shortening of native mitral valve.
Delivery approach: Endovascular venous delivery.
Device size: 12 Fr delivery system.
Procedural details
The procedures were carried out under general anaesthesia with transoesophageal echocardiographic (TEE) guidance. The procedure has been described in detail previously1,2 and is shown in Figure 1.
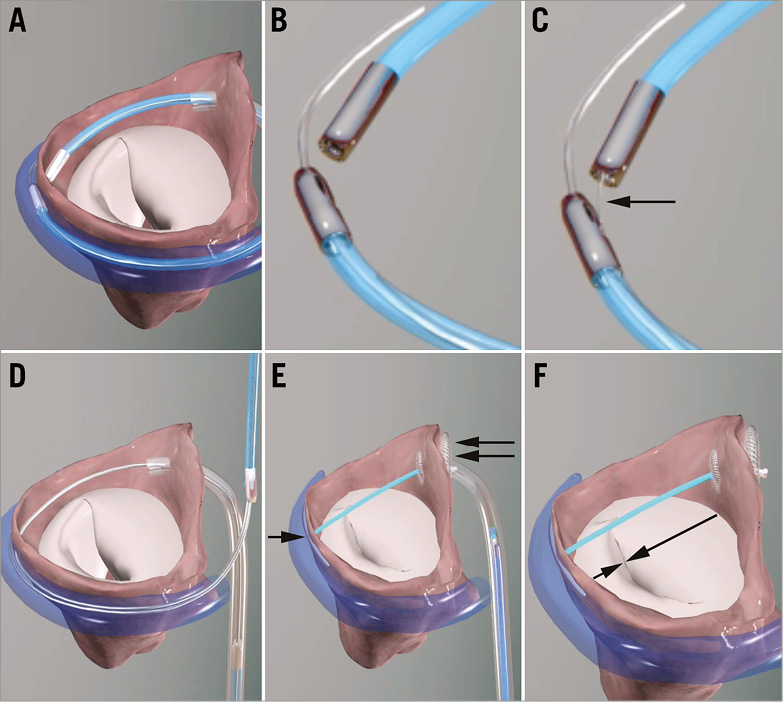
Figure 1. The Arto System implantation procedure. A) Great cardiac vein (GCV) and left atrial (LA) MagneCaths in position and magnetically linked behind the P2 segment of the posterior mitral leaflet. B) Close-up of magnetically linked LA and GCV MagneCaths. Each magnetic catheter has a specific shape and lumen to direct and receive the crossing wire. C) The crossing wire (arrow) is pushed from the GCV into the LA MagneCath. The MagneCaths are aligned to direct the wire safely from the GCV to the LA through the atrial wall. D) After using an exchange catheter, the loop guidewire in place across left atrium. This guidewire directs the placement of the GCV anchor (T-bar) and septal anchor. E) The MVRx System in place before tensioning. T-bar, single arrow; septal anchor, double arrow. F) Tensioning of the bridge results in precise shortening of the mitral annulus anteroposterior diameter (arrows) and elimination of FMR; once the final position is attained, the suture is cut and secured with a suture lock.
Clinical data
Eleven consecutive patients had successful implantation of the Arto System in the MitrAl ValvE RepaIr Clinical Trial (MAVERIC Trial, clinicaltrials.gov identifier NCT02302872). The demographics and outcomes have been published previously3. All patients were deemed to be high surgical risk by the Heart Team, and were symptomatic with 81.8% being NYHA Class III/IV at baseline. There were no procedural safety events in any patient. There were two clinical events within the 30-day follow-up period: (1) one patient underwent uncomplicated surgical drainage of the effusion without recurrence, and (2) one patient had asymptomatic dislocation of the coronary sinus T-bar and underwent successful elective surgical MV replacement. No other clinical events have occurred at subsequent follow-ups.
At six months, MR grade, LV volumes, mitral annular dimensions, and functional status all improved, and were very stable when compared to 30-day values. Pre-procedure FMR was 90% grade 3-4+, and at six months was 80% grade 1-2+. EROA by PISA at baseline was 30.3±11.1, decreasing at six months to 13.7±8.6 mm2, and regurgitant volumes decreased from 45.4±15.0 to 19.9±11.6 ml. LVESVi decreased from 77.5±24.3 to 68.2±28.2 mL/m2 and LVEDVi from 118.7±28.6 to 104.9±30.2 ml/m2 at six months. Mitral annular anteroposterior diameter decreased from 45.0±3.3 to 38.9±2.7 mm. Functional status was 81.8% NYHA Class III/IV and 18.2% Class I/II at baseline, improving at six months to 50.0% Class III and 50.0% Class I/II.
Ongoing studies
Enrolment of up to 20 additional patients at three sites (Riga, Latvia; Massy, France and London, United Kingdom) is expected to begin shortly in Phase II of the MAVERIC trial.
Unique features
Unique features of the Arto System include the relatively short procedure time, as well as the absence of haemodynamic instability or ventricular arrhythmias. Device efficacy is seen immediately at the time of implantation, and the system can be adjusted by tensioning or relaxing the suture element prior to device lock and release. The Arto System is recapturable and retrievable during the deployment.
Potential improvements
With additional patient outcomes data, the preferred placement of the anchors for a given anatomy as well as the preferred amount of A-P shortening will be known and result in maximising benefits to the patient. System improvements include the addition of new anchor designs and streamlining of the catheter delivery system.
Conflict of interest statement
J. Rogers, M. Thomas, and S. Greene are consultants to MVRx. A. Erglis has an indirect equity interest in MVRx. The other authors have no conflicts of interest to declare.

