Introduction
Along with aortic valve stenosis, mitral regurgitation (MR) accounts for most of the acquired valvular disorders encountered in adulthood1. From a purely pathological perspective, MR is organic (primary) or functional (secondary) in nature2. As opposed to organic MR, the intrinsic mitral valve components remain at least theoretically intact in functional MR. However, in the majority of patients, elements of organic and functional MR collectively present3.
Cardiology societies on both sides of the Atlantic have issued guidelines on how to approach patients with severe MR4,5. Nonetheless, data from the EURO Heart Survey, as well as US-based studies, suggest that up to one half of patients with severe mitral regurgitation are not referred for mitral valve surgery because of age, impaired left ventricular function and/or comorbidities6,7. General consensus indicates that surgical mitral valve repair, whenever feasible, is the preferred strategy; unfortunately, expertise in mitral valve repair is not widely spread and remains restricted to specific centres of expertise.
The last decade has seen a surge in innovative transcatheter concepts to address MR8. These alternatives are potentially appealing as they are truly minimally invasive, do not require cardiopulmonary bypass and might be executed under conscious sedation. One concept currently under clinical evaluation utilises the proximity of the coronary sinus to the left atrioventricular junction (i.e., the virtual mitral annulus) to create an indirect mitral annuloplasty. This technical report illustrates mitral annuloplasty with the Viacor percutaneous transvenous mitral annuloplasty (PTMA®) system (Viacor Inc, Wilmington, MA, USA).
Technical specifications
Description
The PTMA system is a sterile, single operator implantable cardiac device with essentially two customised implant components: an implant catheter and customised rods placed internal to the implant catheter (Figure 1, Moving image 1). Since the initial clinical experience in 2006, several device iterations have led to the current implantation system.
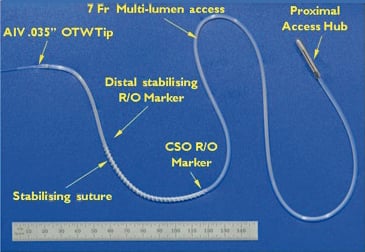
Figure 1. PTMA device.
The 7 Fr Teflon (Poly-Tetra-Fluoro-Ethylene) PTMA implant catheter is an over-the-wire (0.035” guidewire compatible) system, comes in lengths of 55 and 65 cm (with 3 cm distally extended tips available for patients with especially enlarged hearts to ensure catheter placement well into the anterior interventricular vein), and contains three parallel lumina accommodating up to three rods of equivalent length and stiffness (one rod per lumen) (Figure 1, Moving image 1). Its distal portion consists of a single lumen extrusion (18mm) providing a low profile, a-traumatic, trackable tip, which transitions to the full diameter of the multi-lumen extrusion. Along the outer surface of the designated segment extending from the coronary sinus ostium to the midpoint of the coronary sinus-great cardiac vein arc (corresponding to the P2 segment of the mitral annulus), a traction element consisting of 2-0 non-absorbable polyester surgical suture (76mm in length) is woven around the catheter to provide additional grip to the vessel wall, thereby increasing device stability. Radiopaque markers on both ends of the suture as well as one marker at the distal end of the catheter assist in placing the device in the correct anatomical position. The proximal end of the implant catheter consists of a titanium hub enabling controlled telescoping of the implant rods and subsequent secured positioning of the rods within the implant catheter for precise and optimal anatomic positioning.
The 0.035’’ rods are composed of nitinol (binary nickel-titanium alloy), ranging in overall length from 50.7 to 66.7cm.The distal treatment segments come in various lengths (110, 120, 130, 145 and 160 mm) to accommodate patient-specific anatomy and deliver 200g/cm of stiffness to reduce the anterior-posterior dimension of the mitral annulus.
Coronary venous anatomy
The coronary sinus is the principle venous confluence of the heart. Its orifice is located in the lower postero-septal third of the right atrium, superior and medial to the inferior caval vein opening, inferior, medial and slightly more anterior to the fossa ovalis, and lateral and posterior to the hinge point of the septal tricuspid leaflet9. The Thebesian valve, a semicircular membranous fold, guards this orifice. The coronary sinus courses epicardially through the posterior left atrioventricular groove at the base of the heart, tapering down in size and turning into the anterior atrioventricular groove to proceed as the great cardiac vein. As it deviates into the anterior interventricular groove it becomes the anterior interventricular vein. The coronary sinus runs in relative proximity to the left atrioventricular junction (so-called mitral annulus). In the majority of cases, the body of the sinus is adjacent to the inferior left atrial wall, about 10mm above and cranial to the deeper mitral annulus (Figure2). The anatomical relationship between both structures changes along the course and shows considerable individual variability9. In general, the coronary sinus is closest to the lateral segment of the mitral annulus at the midpoint of the posterior/mural mitral leaflet corresponding to the level of P2.
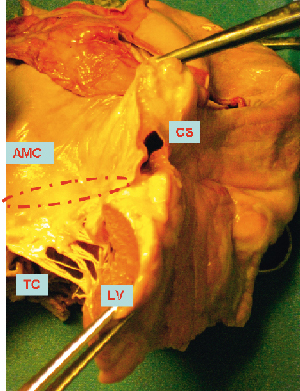
Figure 2. Pathology specimen illustrating the coronary sinus (CS) well above the “mitral annulus” (MA). LV: left ventricle; TC: tendinous cords; AMC: aortic-mitral curtain
Concept
By its relative proximity to the mitral annulus (i.e., the left atrioventricular junction) the coronary sinus can be used to perform transcatheter indirect mitral annuloplasty. A dedicated endoluminal device with the ability to provide anterior-posterior annular reduction is advanced into the coronary sinus (Figures 3 and 4). With the Viacor PTMA system, the implant catheter is introduced through the left or right subclavian vein, passing the superior caval vein and the right atrium into the coronary sinus10. The most distal portion of the catheter extends into the anterior interventricular vein so as to provide full coverage of the entire atrioventricular groove, ensuring that the pressure from the rods, once introduced, is supported by the fibrous structure of the septum. An anterior push is built up by advancing up to three nitinol rods in the delivery catheter. The device produces diffuse outward force across its proximal and distal ends, resulting in anterior displacement of the middle scallop of the mural leaflet and a decrease in the septal-to-lateral diameter of the valve (Figure 5).
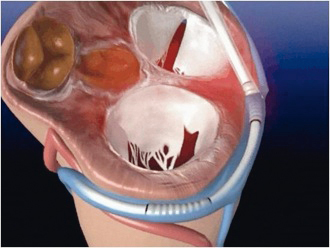
Figure 3. Cartoon illustrating PTMA device in the coronary sinus surrounding the posterior segment of the left atrioventricular junction (“annulus”).
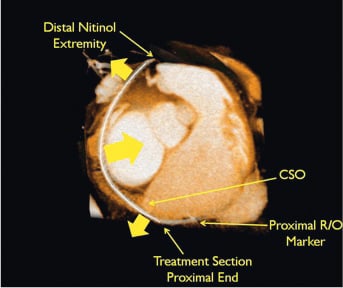
Figure 4. MSCT image illustrating the PTMA device in situ. Courtesy of Christoph Hehrlein, MD, PhD, University of Freiburg, Investigator, PTOLEMY 2 study.
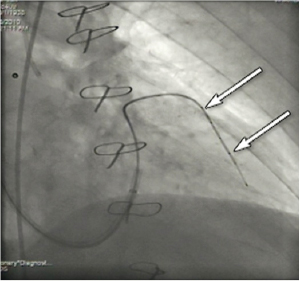
Figure 5. Magic wire into the anterior interventricular vein. Arrows identify the markers.
Implantation step-by-step
Following sequential product and technique iterations since 2006, PTMA implantation has shifted from a staged procedure initially utilising diagnostic tools to determine optimal device sizing to a direct-to-implant technique employing a wider spectrum of PTMA sizes, resulting in improved technical success and reduced procedure times.
Pre-procedurally, a MSCT scan is undertaken to confirm coronary sinus –great cardiac vein– anterior interventricular vein patency, determine the location of coronary artery crossover and optimally size both the implant catheter and nitinol rods.
The implantation procedure commences with the patient under either conscious sedation or general anaesthesia, with continuous arterial pressure and electrocardiographic monitoring. Conventional angiography of the left coronary artery is performed for appreciation of the left coronary artery, with focus on the proximal segments of LAD, LCX and tributaries at baseline and at the end of the procedure. This is followed by occlusive venography to establish landmarks and facilitate access to the distal venous anatomy. Subclavian vein access (right or left side), cannulation of the coronary sinus, wiring of the anterior interventricular vein, advancement of the over-the-wire delivery catheter, introduction of up to three nitinol rods, fixation of the total assembly to the subclavian fascia and closure of the access pocket subsequently follow and are now described in further detail.
Subclavian venous access is obtained with a commercial peel-away 8Fr or 9Fr coronary sinus access sheath (e.g., Pressure Products; Pressure Products, Inc.,San Pedro, CA, USA or Biotronik ScoutPro; Biotronik, Berlin, Germany) using the Seldinger technique at the transition level of the medial to middle third of the collarbone. Too medial access might impede proper catheter manipulation and even result in future device fracture, as the system would risk entanglement between the first rib and the collarbone.
After successful cannulation of the coronary sinus, a conventional 0.014” coronary guidewire is introduced into the great cardiac vein – anterior interventricular vein crossover. Thereafter an occlusive coronary sinus venogram in two orthogonal angiographic views (typically RAO 30° and LAO 30°- caudal 30°) is made using a wedge balloon catheter. Subsequent simultaneous left coronary artery and coronary sinus angiography offers a detailed appreciation of the spatial relationship between the coronary sinus and left coronary artery (provided flow-through is adequate) and can be repeated at any time during the procedure at the operator’s discretion (Moving image 2).
The 0.014” guidewire is then advanced 3-5 cm distally into the descending anterior interventricular vein. The wedge balloon catheter is switched out for a 5 Fr or 6 Fr 100 cm exchange Terumo Glidecath (Terumo Corp., Tokyo, Japan), which is advanced into the descending anterior interventricular vein. The 0.014’’ guidewire is then exchanged for a stiffer 0.035’’ 260cm Boston Scientific Magic Torque (Boston Scientific, Natick, MA, USA) coronary guidewire (Figure 5, Moving image 3).
The Glidecath is then removed and the 8 Fr coronary sinus access sheath is replaced with the 9 Fr 50cm Pressure Products peel-away non-braided SafeSheath CSG Worley, which is advanced over the Magic Torque wire to the midpoint of the great cardiac vein.
With a stiffening obturator in one of its three lumina, the PTMA implant catheter is advanced over the Magic Torque wire landing its distal tip in the descending portion of the anterior interventricular vein and its proximal marker at the junction of the posterior interventricular vein and coronary sinus ostium (Figure 6, Moving image 4). Crossing the great cardiac vein into the anterior interventricular vein can be challenging and requires a controlled push-and-pull manipulation of both the guide catheter and PTMA implant catheter while simultaneously peeling away the coronary sinus sheath. The remaining unpeeled, proximal end of the coronary sinus sheath is clamped to re-establish haemostasis. The first nitinol rod is now introduced in the open lumen of the PTMA delivery catheter (one lumen contains the obturator, the other the Magic Torque wire) (Moving image 5). Application of surgical-grade silicon oil on the rods and a dedicated rod advancement tool facilitate this manoeuvre. The obturator is then replaced by a second nitinol rod of the same length and stiffness as the first introduced rod. Next, the Magic Torque wire is exchanged for the third and final rod, ensuring the PTMA implant catheter positioning is maintained relative to the previously demarcated landmarks. With the introduction of each additional rod, one can evaluate the therapeutic effect and crescendo forward push of the mitral annulus by fluoroscopy and echocardiography, readjusting if needed (Figure 7, Moving image 6). A titanium cap is applied to the proximal titanium hub of the PTMA implant catheter to encapsulate the proximal ends of the nitinol rods. Using a dedicated pullback tool, the rods internal to the catheter can be moved up to 25mm proximally to achieve optimal positioning relative to the venous anatomy and optimise treatment effect (Figure 8, Moving image 7). After angiographic exclusion of coronary artery impingement and vasospasm, the cap and hub are locked and the remainder of the CSG sheath is peeled away. A housing pocket is created underneath the surrounding muscle and fascia. The proximal hub of the PTMA system is fixated to the fascia with non-absorbable 2-0 sutures. The skin is closed in layers and the small incision is draped in sterile fashion.
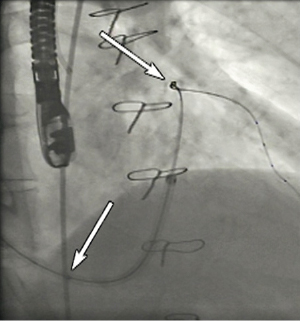
Figure 6. PTMA device in situ; lower arrow marks the proximal marker at the ostium of the coronary sinus, the upper arrow marks the distal marker at the level of the descending portion of the anterior interventricular vein.
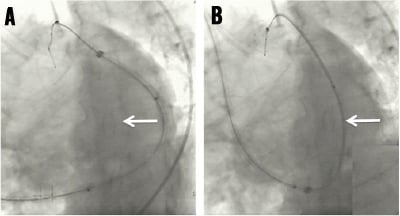
Figure 7. PTMA device generating anterior push of the posterior segment of the “mitral annulus”.
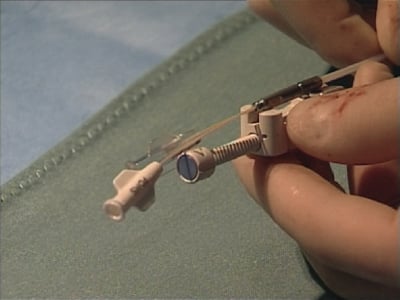
Figure 8. Dedicated device for final positioning of the rods in the PTMA catheter over a maximal range of 25 mm.
Percutaneous retrieval of the PTMA device is possible at any given post-procedural stage. After opening the subclavicular housing pocket, the proximal hub is opened and the rods are withdrawn one after the other. Afterwards, the PTME catheter can be pulled out of the subclavian vein. Notably, the mitral valve apparatus remains accessible for potential surgical repair10, as no alteration to the leaflets or apparatus occurs when the active PTMA system is removed from the patient.
Indication for use
In the heart failure population, the importance of pharmacological neurohormonal modulation (adrenergic system and renin-angiotensin-aldosterone axis) in general, and device-oriented therapy (automated implantable cardiac defibrillator, cardiac resynchronisation therapy) in selected patients, is well established by evidence-based medicine11,12. Despite these therapeutic options many heart failure patients remain symptomatic. A significant portion will have at least moderate to severe functional mitral regurgitation with some degree of preserved integrity of the mitral valve leaflets. Surgical mitral valve repair in this group of patients is controversial, with several registries demonstrating conflicting results13. Furthermore, the surgical expertise to perform high-quality and durable mitral valve repair is not widespread. The concept of indirect transcatheter mitral annuloplasty through the coronary sinus targets patients with heart failure and at least moderate functional MR in NYHA class 2-4 despite optimal medical therapy.
Initial experience
The technical feasibility and proof of concept with the PTMA mitral annuloplasty system was first demonstrated in an ovine model of ischaemic cardiomyopathy with functional MR: reduction in MR was achieved by reducing the septal-to-lateral mitral annular dimensions and MV tenting area14,15. These results were duplicated in human subjects with symptomatic ischemic MR grade 2-4 who were scheduled for conventional surgical mitral valve repair16. In three out of four patients a “temporary” PTMA device was successfully implanted percutaneously with clear reductions in septal-to-lateral mitral annular dimensions and mitral regurgitant volume. Subsequently, the Percutaneous TransvenOus Mitral AnnuloplastY System to reduce Mitral Valve Regurgitation in patients with Heart Failure (PTOLEMY-1 trial), a multicentre study to assess the safety and feasibility of a permanent PTMA implant device in 27 symptomatic heart failure patients (NYHA class 2-3) with moderate to severe functional MR10, was conducted to evaluate the first-generation PTMA system. In 13 out of 19 patients who underwent a diagnostic PTMA study, device stability and reductions in mitral annular dimensions and MR severity by at least 1 grade were documented by TEE (MR grade 3.2±0.6 reduced to 2.0±1.0) without detrimental effects on coronary arterial flow; these 13 patients were eligible for an attempt to implant the permanent PTMA device which turned out successful in nine cases. There was one case of pericardial effusion, one transient renal dysfunction and one coronary circumflex artery spasm requiring percutaneous coronary intervention. There were no permanent procedure or device related major adverse events. During follow up percutaneous device removal occurred in four patients (on days 7, 84, 197, and 216 post implantation): two cases of device migration and one case of device fracture and insufficient efficacy. Three of these patients proceeded with uneventful conventional surgical mitral annuloplasty. At 3-month follow up, 3-D echo confirmed sustained reductions of mitral annulus septal-lateral dimension (4.0±1.2 mm), but only modest impact on the MR severity.
The PTOLEMY-2 safety and efficacy study, utilising an improved PTMA system and technique, enrolled patients at 16 sites in Europe and Canada. Preliminary data on the first 39 enrolled patients with attempted PTMA implantation in 35, revealed procedural success in 28 (80%) with core lab adjudicated composite quantitative MR relative reductions of 20% in one-third of patients and 40% in another one-third of patients at six months. In approximately half of the patients, treatment effect only became noticeable three to six months after the index procedure, indicating that the active nature of the nitinol PTMA device imparts continual and positive forces in reshaping the MV anterior-posterior diameter.
Conclusion
The Viacor PTMA system is a transcatheter design to achieve indirect mitral annuloplasty in patients with functional MR. During the PTMA implantation procedure, real time monitoring of progressive mitral annular reduction and coronary arterial flow state is achieved by conventional coronary angiography and TOE. At any given moment the device can be easily retrieved, and as annuloplasty of the MV is achieved in a-traumatic fashion, the mitral apparatus remains accessible for surgical mitral repair if clinically indicated.
Acknowledgement
The authors are grateful to Professor S. Sack (Städtisches Klinikum München, Germany) for his pioneering work in the field of transcatheter indirect mitral annuloplasty and his excellent proctoring at our centre.
Conflict of interest statement
R. Spencer is an employee of Viacor Inc. The other authors have no conflict of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

