Abstract
Aims: The pivotal ATLANTA first-in-man study showed the promising safety and efficacy profile of the novel Catania™ stent in a population with ~20% American College of Cardiology/American Heart Association (ACC/AHA) type C coronary lesions. The ATLANTA 2 registry was designed to evaluate the 12-month safety and efficacy of the Catania stent in a broader real world scenario.
Methods and results: The ATLANTA 2 registry was a prospective, non-randomised, single-arm study of patients with symptomatic ischaemic heart disease and de novo lesions of native coronary arteries. A total of 300 patients (396 lesions) were recruited and 482 Catania stents were implanted. At 12 months, major adverse cardiac events were 8.8%, mainly driven by target lesion revascularisation (6.5%). Cardiac death and non-fatal myocardial infarction occurred in 2.5% and 0.7% of patients, respectively. Subacute definite or probable stent thrombosis was 0.7%. No late stent thrombosis was recorded. Compared with patients treated with drug-eluting stents or bare metal stents in the study period, those treated with Catania stents experienced similar outcomes at one year.
Conclusions: The 12-month results of the ATLANTA 2 registry confirmed the positive results of the ATLANTA first-in-man trial in a more complex population. A randomised trial is needed to assess the comparative value of the Catania stent over currently-used drug-eluting stents or bare metal stents.
Introduction
Drug-eluting stents (DES) reduce the formation of neointimal hyperplasia, a proliferative maladaptive healing response to stent implantation with the potential for restenosis and repeat revascularisation1-3. However, safety issues relevant to DES use in real world scenarios have been raised over their higher rates of malapposition, wall inflammation, hypersensitivity reactions, lack of or delayed endothelialisation and late stent thrombosis compared with bare metal stents (BMS)4. As a result, DES require long-term dual antiplatelet therapy, with attendant increased costs and risk of severe bleeding. These shortcomings of DES have encouraged the development of biologically based platforms, including BMS covered with biomimetic coatings5.
The Catania stent (CeloNova Biosciences, Newnan, GA, USA) has a cobalt-chromium alloy with a nano-thin poly(bis[trifluoro-ethoxy])-phosphazene surface modification featuring anti-inflammatory, pro-healing and anti-thrombogenic qualities6. In the Assessment of The LAtest Non-Thrombogenic Angioplasty stent (ATLANTA) first-in-man (FIM) trial, the Catania stent was associated with a0.60mm angiographic late luminal loss and 99.5% healing by optical coherence tomography at six months, no reported death or myocardial infarction, and a clinically-driven target lesion revascularisation (TLR) rate of 3.6% at 12 months7,8. No stent thrombosis was reported, despite dual antiplatelet therapy being prescribed for only one month7.
After having received CE (Conformité Européenne) marking, experience with the Catania stent now extends to patients outside the inclusion criteria of the controlled FIM trial. ATLANTA 2 was a single centre, prospective, non-randomised registry run to provide insights into the performance of the Catania stent in a broader population of patients than those enrolled in the ATLANTA-FIM trial with diverse characteristics and more complex clinical or angiographic presentations.
Materials and methods
Study population
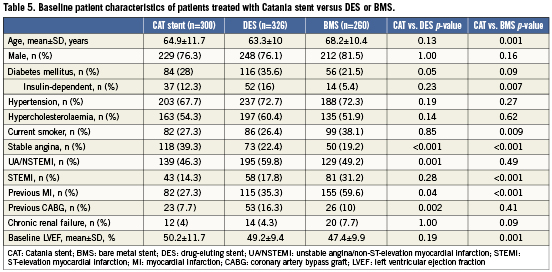
A total of 300 patients with coronary artery lesions suitable for stenting with a BMS and for whom implantation with a Catania stent was at the operator’s discretion, were enrolled between May 2007 and March 2008. The registry population included a large proportion of patients with clinical or angiographic characteristics that represented exclusion criteria in the pivotal ATLANTA-FIM trial, such as age >70 years old, life expectancy <1 year, chronic renal failure defined as creatinine >2mg/dL, ongoing acute myocardial infarction or myocardial infarction within the last 48 hours, left ventricular ejection fraction <30%, cardiogenic shock, antithrombotic drugs intolerance, cardiac and/or extracardiac documented disease requiring surgical or percutaneous repair, vessel size <2.5 or >3.5mm, lesion length >20mm, planned >2 stents implantation, visible endocoronary thrombosis, extreme vessel tortuosity, diffuse and severe calcifications, unprotected left main, bifurcation, bypass graft and/or chronic total occlusion. Baseline characteristics and outcomes of patients treated with Catania stents were also retrospectively compared with those of patients referred to DES (either sirolimus- or paclitaxel-eluting stents) or other BMS implantation in the study period.
The study was conducted according to the Declaration of Helsinki. The medical ethics committee approved the study protocol. Written informed consent was obtained from all patients.
Interventional procedure
Interventional procedures and adjuvant therapies were performed according to international guidelines9. Before the procedure, patients were prescribed aspirin (75 to 300mg) and clopidogrel (600mg loading dose). During the procedure, all patients received intravenous unfractionated heparin (50 to 150IU/Kg) to maintain the activated clotting time between 250 and 300 seconds. Glycoprotein IIb/IIIa inhibitors were used at the physician’s discretion. All patients were advised to maintain aspirin lifelong (100mg/day) and thienopyridine for at least four weeks (or up to 12 months in case of acute coronary syndrome, at the physicians’ discretion). Predilatation or direct stenting were performed at the discretion of the operator. Angiographic optimisation was performed by high-pressure dilation to achieve <10% residual stenosis by visual estimate after stent implantation.
Follow-up and study endpoints
All clinically relevant baseline variables were recorded on electronic case report forms. Follow-up data were prospectively collected at 30 days, six months and 12 months and consisted of telephone or in-office assessment. Referring cardiologists, general practitioners and patients were contacted whenever necessary for further information. All repeat revascularisation and re-hospitalisation data were prospectively collected during follow-up and entered into the centralised computer system of our institution or by directly contacting the hospitals where the patients were admitted or referred. Clinical events were adjudicated by an independent clinical events committee.
The primary objective of the ATLANTA 2 registry was the 12-month rate of major adverse cardiac events (MACE). MACE were defined as the composite of cardiac death, non-fatal myocardial infarction (MI) or TLR. Cardiac death was defined as any unexplained sudden death or death due to acute myocardial infarction, ventricular arrhythmia or refractory heart failure. MI (excluding periprocedural MI) was defined as creatine kinase-MB enzyme elevation ≥3 times the upper limit of the normal value. TLR was defined as any clinically-driven repeat percutaneous revascularisation or surgical bypass of the original target lesion site. Secondary objectives were the 12-month rates of all-cause death, cardiac death, non-fatal MI,TLR, target vessel revascularisation (TVR, defined as any clinically-driven repeat percutaneous revascularisation or surgical bypass of the original target vessel, either percutaneous or surgical), TVR non TLR, the composite of cardiac death and non-fatal MI, the composite of all-cause death, non-fatal MI or TVR, and the composite of definite or probable stent thrombosis at 30 days and 12 months, according to the Academic Research Consortium definitions10.
Statistical analysis
Continuous variables were analysed for a normal distribution with the Shapiro-Wilk test. Continuous variables following a normal distribution are presented as mean ± standard deviations and were compared using the Student’s unpaired t test. Variables not following a normal distribution are expressed as median (interquartile range) and were compared with the Mann-Whitney rank sum test. Categorical variables are presented as counts and percentages and were compared by means of the chi-square test or Fisher’s exact test when at least 25% of values showed an expected cell frequency below five. The time to MACE was summarised by using the Kaplan-Meier method. Patients lost to follow-up were considered at risk until the date of last contact, at which point they were excluded. The log rank test was used for comparison between Kaplan-Meier estimates. Control for potential confounders when comparing the outcomes of patients treated with a Catania stent versus those treated with a DES or BMS was attempted by using a Cox regression model. The variables entered into the model for statistical adjustment were those listed in Tables1 and 2 that were considered clinically relevant or presented with a p-value <0.20 in the univariate analysis. Results are expressed as adjusted hazard ratio (HR) and 95% confidence interval (95% CI). All probability values reported are two-sided, and a value of p<0.05 was considered to be significant. Statistical analysis was performed using PASW version 18.0 software (SPSS Inc., Chicago, IL, USA).
Results
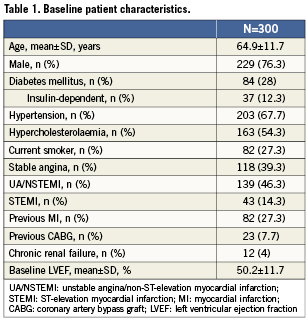
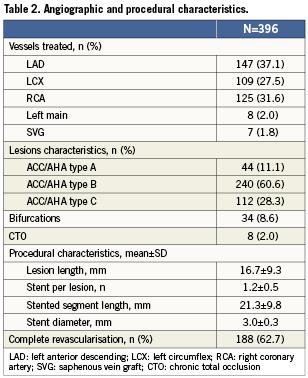
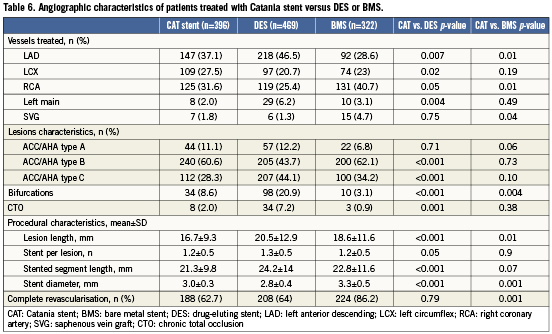
A total of 300 patients were recruited to the ATLANTA 2 registry, and a total of 396 lesions were treated with 482 implanted stents. Twelve-month follow-up data were available in 279 (93%) of patients. Patient and lesion characteristics are summarised in Tables1 and 2. The mean age was 64.9±11.7 years and 77% of patients were men. Twenty-eight percent of patients had diabetes mellitus, 4% had chronic kidney disease and 60.6% presented with an acute coronary syndrome. Of the lesions treated, 165 (41.7%) were classified as American College of Cardiology/American Heart Association type B2 or C. The left anterior descending artery was the target vessel in 37.1% of cases. The average lesion and stent lengths were 16.7±9.3mm and 21.3±9.8mm, respectively. Small vessels were substantially under-represented with a final mean stent diameter of 3.0mm. The mean number of stents implanted per patient was 1.6±0.8.
After the procedure, at baseline, 98% of patients were taking dual antiplatelet therapy with aspirin plus clopidogrel or ticlopidine, 1.3% were taking aspirin alone, 0.3% were taking clopidogrel alone and 0.3% were not taking any antiplatelet drug. Patients on dual antiplatelet therapy at four weeks and 12 months were 97.3% and 18.3%, respectively.
Clinical outcomes
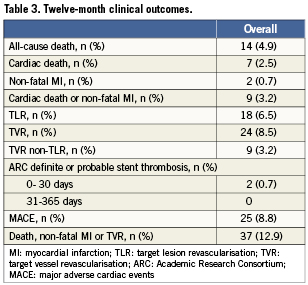
At 12 months, MACE in the overall cohort were 8.8% (Table3). This rate was mainly driven by TLR, which occurred in 6.5% of the entire population. Conversely, cardiac death and non-fatal MI occurred in 2.5% and 0.7% of patients, respectively. At 30 days, the definite or probable stent thrombosis rate was 0.7%. No late thrombosis were recorded up to 12 months.
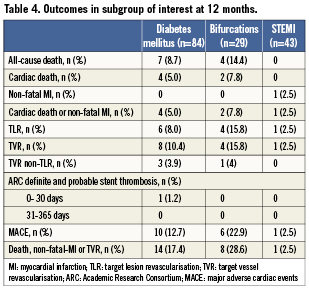
Clinical outcomes at 12 months in subgroups of interest are reported in Table 4. MACE occurred in 12.7%, 22.9% and 2.5% of patients with diabetes mellitus, bifurcation lesions and STEMI, respectively.
Catania stent versus DES or other BMS
Tables 5 and 6 show baseline and angiographic characteristics of patients treated with the Catania stent versus those treated with DES (n=326) or other BMS (n=260) in the study period. Compared with those who received a Catania stent, patients treated with DES were more likely to present with acute coronary syndromes, history of previous MI and coronary artery bypass graft. Not surprisingly, patients treated with DES also presented with higher coronary complexity, including a higher prevalence of bifurcations, chronic total occlusions, small vessels and long lesions. Compared with patients treated with Catania stents, those who received BMS were older and presented more frequently with STEMI and lower left ventricular ejection fraction. However, BMS patients had larger vessels and lower rates of bifurcations compared to Catania stent patients.
At one-year the crude unadjusted outcomes were similar among patients who received Catania stents, DES or BMS (Figure1). After adjustment for potential confounders, Catania stents did not differ significantly from DES in terms of MACE (HR 1.20; 95% CI 0.65-2.22; p=0.57), cardiac death (HR 2.10; 95% CI 0.52-8.45; p=0.30), MI (HR 0.95; 95% CI 0.12-7.45; p=0.96) or TLR (HR 1.3; 95% CI 0.63-2.72; p=0.47). Similarly, there were no significant differences between Catania stents and BMS either in terms of MACE (HR 0.93; 95% CI 0.45-1.93; p=0.84), cardiac death (HR 1.73; 95% CI 0.28-10.70; p=0.56), MI (HR 1.27; 95% CI 0.16-10.27; p=0.82) or TLR (HR 0.70; 95% CI 0.31-1.59; p=0.40).
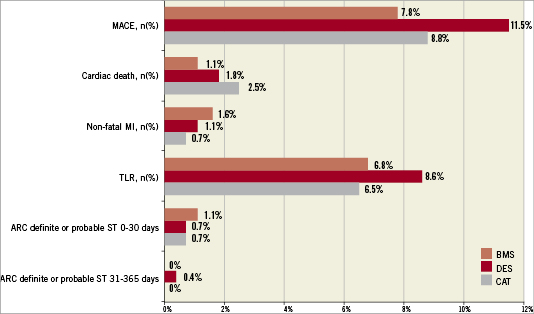
Figure 1. One-year outcomes of patients treated with Catania (CAT) stents compared with patients who received drug-eluting stents (DES) or other bare metal stents (BMS) in the study period. ARC: Academic Research Consortium; MACE: major adverse cardiac events; MI: myocardial infarction; TLR: target lesion revascularisation; ST: stent thrombosis; All p values: NS
Discussion
Unsolved concerns associated with the use of both BMS (e.g., restenosis) and first generation DES (e.g., stent thrombosis, need for prolonged dual antiplatelet therapy with attendant increased costs and bleeding risk) have shifted the industry interests towards the development of novel categories, such as biodegradable stents, new durable polymers, biodegradable polymers, polymer-free DES and stents with novel coatings5. Among the latter, the Catania stent features unique anti-thrombogenic and anti-inflammatory properties ascribable to a ~40nm thick coating of Polyzene-F, an inorganic rubber that has been shown to be associated with preferential adsorption of albumin, low distortion of protein structures and low platelet adhesion and activation6.
Following the promising results of the first-in-man investigation7, the prospective ATLANTA 2 registry was designed to evaluate the one-year safety and efficacy of the Polyzene-F covered Catania stent in a larger and more complex population. Although the study obviously suffers from the typical limitations of non-randomised studies, it usefully contributes to generating some hypotheses. In particular, the study results might suggest a role for the Catania stent as a suitable alternative to BMS in routine clinical practice. This hypothesis deserves further testing in a randomised controlled clinical trial or a cost-effectiveness analysis. Importantly, the ATLANTA 2 registry results are similar to those obtained in the ATLANTA FIM study, despite the presence of a substantial proportion of patients presenting more complex clinical and angiographic characteristics than those allowed in the FIM trial.
Outcome data were available for 93% of patients, a rate which compares favourably with other published registries11-13. Angiographic follow-up, which is known to accentuate the rates of repeat revascularisation compared with clinical follow-up alone, was not routinely recommended in this study, which focused on clinical endpoints. Careful monitoring of patients was performed to ensure high data quality and accurate reporting of events. At 12months, MACE were reported in 8.8% of patients, mainly driven by TLR, a rate which compares favourably with those observed at one year with BMS and is not far from those reported for selected types of DES13-15. Importantly, in our adjusted comparison versus DES or BMS implanted in the study period, the Catania stent did not show relevant differences compared to the other groups in terms of safety and efficacy. However, these comparisons were not randomised and statistical adjustment might not capture entirely the presence of assessed and unassessed confounders. In particular, although the Catania stent population included lesions at high risk for restenosis and need for repeat revascularisation, small vessels were substantially under-represented as shown by a mean stent diameter of 3mm. Also, as per our routine practice during the study period, the Catania stent was preferentially used in patients at high risk of bleeding or with life expectancy <1 year, due to the low rates of stent thrombosis seen in the ATLANTA FIM trial with no need for dual antiplatelet therapy beyond one month. Therefore, a number of baseline differences in patients’ characteristics may have biased the comparison among stents, which is provided for exploratory purposes and should be interpreted with caution.
As in the ATLANTA FIM trial, safety represents one of the most attractive features of the Catania stent. Cardiac death and MI were registered in only 2.5% and 0.7% of patients, respectively, at 12 months. Definite or probable stent thrombosis occurred early in two patients (0.7%), while no late event was recorded. This compares favourably with the 12-month data of the Endeavor® zotarolimus-eluting stent (Medtronic, Minneapolis, MN, USA) and XIENCE® everolimus-eluting stent (Abbott Vascular, Redwood City, CA, USA) in real world clinical practice, reporting stent thrombosis rates in the range of 0.2%-1.1%13,16,17. Importantly, these outcomes were obtained with only one month of dual antiplatelet therapy in the vast majority of patients. These findings could suggest a role for the Catania stents in selected scenarios, such as those in which aBMS would be preferred over a DES because of a high risk for stent thrombosis (e.g., acute myocardial infarction) or a high risk for bleeding (e.g., elderly, patients undergoing non-cardiac surgery).
The most important limitation of this study, as mentioned before, is the lack of a randomised comparison with a different stent. Such a comparison is key to determining specifically whether unfavourable outcomes are related to the clinical status of the patients, rather than the use of these devices per se. Another caveat is that patients were not enrolled consecutively. However, the clinical and angiographic characteristics of the study population reflect those of a general population of patients treated with BMS. In addition, we provided data for patients receiving DES and BMS in the study period.
Conclusions
The 12-month results of the ATLANTA 2 registry confirmed the positive results of the ATLANTA first-in-man trial in a more complex population. An appropriately-powered randomised trial is needed to assess the comparative value of the Catania stent over currently used DES or BMS.
Acknowledgements
The authors would like to acknowledge in this paper the Associazione Cuore e Ricerca, Catania, Italy.
Conflict of interest statement
The authors have no conflict of interest to declare.

