Abstract
Aims: Residual thrombus accumulation around stent struts has been observed after the end of primary PCI and may represent a risk factor for acute stent thrombosis. The aim of this study is to test whether a strategy of prolonged bivalirudin infusion may reduce thrombosis of stent struts as compared to an intraprocedural only administration in subjects undergoing primary PCI.
Methods and results: One hundred and sixty patients will be selected from the MATRIX (Minimizing Adverse Haemorrhagic Events by TRansradial Access Site and angioX) study with all the following inclusion criteria: a) STEMI patients undergoing primary PCI with stent implantation, b) randomisation to standard or prolonged bivalirudin treatment arm, and c) at least one critical (>70%) stenosis of non-infarct-related coronary vessels suitable for staged PCI. Optical coherence tomography (OCT) of the infarct-related artery will be performed at the end of primary PCI and at the time of staged PCI which will be performed three to five days after the index procedure. The percentage difference in minimal flow area and in the number of stent cross-sections with a thrombotic area >5% will be measured at the end of primary PCI and at the time of staged PCI.
Conclusions: The MATRIX OCT substudy will establish whether prolonging the infusion of bivalirudin after the end of primary PCI may reduce the amount of residual thrombotic material on stent struts. The effect of the long-infusion strategy on clinical outcome will be elucidated by the MATRIX study.
Rationale of the study
Primary percutaneous coronary intervention (PPCI) is well established as the treatment of choice for patients presenting with acute ST-elevation myocardial infarction (STEMI), leading to an improved patency rate of the infarct-related artery, greater myocardial salvage and, ultimately, to an improved survival as compared to conventional thrombolytic treatment. Coronary thrombosis plays a dual role in the pathophysiology of ST-elevation acute coronary syndrome (STEACS): besides the occlusion of the epicardial coronary vessel, distal embolisation of thrombotic debris may occur, either spontaneously or during mechanical treatment, resulting in obstruction of the microcirculation and ultimately exacerbating myocardial damage1,2. Despite the use of aggressive antithrombotic therapies3-5 and devices engineered to allow for intraprocedural thrombus removal6, residual thrombus burden within the infarct-related artery has been observed repeatedly with optical coherence tomography (OCT) at the end of PCI7. Magro et al showed that residual thrombosis of stent struts is detectable in 100% of subjects undergoing primary PCI and, the greater the thrombotic burden, the worse the myocardial reperfusion was8. Furthermore, residual thrombosis of stent struts may promote further thrombus growth and may represent a risk factor for acute stent thrombosis. Hence, current evidence suggests that the extent of residual in-stent thrombus material is an independent marker of adverse outcome during and/or after PCI and that specific interventions are desirable to reduce its occurrence/extension effectively.
Bivalirudin is currently regarded as the antithrombotic of choice in the setting of primary PCI9. As compared to the combination of unfractionated heparin (UFH)+glycoprotein IIb/IIIa inhibitor (GPI), the use of bivalirudin has demonstrated a reduction in all-cause and cardiovascular mortality at 30 days, which was maintained up to three years. On the other hand, an increase in acute, but not subacute stent thrombosis was also noted in the HORIZONS-AMI trial10-12. In order to overcome this relatively rare yet potentially fatal adverse event, a prolonged infusion regimen of bivalirudin well after PCI has been proposed. In patients undergoing PCI because of either stable or unstable angina, an extended bivalirudin infusion regimen was associated with a lower periprocedural myocardial infarction as compared to intraprocedural only administration13. A subsequent study from the same group demonstrated that a strategy of long-term bivalirudin infusion after PCI in STEMI subjects was non-inferior to the combination of UFH+GPI in terms of myocardial reperfusion14. More recently, the EUROMAX study, which mandated the use of prolonged bivalirudin infusion after PCI in the bivalirudin arm and compared this treatment strategy with UFH plus or minus GPI, showed a reduction of the primary endpoint consisting of death from any cause or bleeding events. However, this composite benefit came at the expense of an increased rate of acute, but again not subacute, stent thrombosis, which was largely interpreted as evidence that a prolonged post-PCI bivalirudin infusion does not offset the hazard of acute ST when bivalirudin is employed in the setting of a PPCI.
The aim of this multicentre and multinational pre-specified MATRIX (Minimizing Adverse Haemorrhagic Events by TRansradial Access Site and angioX) substudy is to test whether the use of a prolonged bivalirudin infusion well after PPCI, as compared to a purely intraprocedural bivalirudin regimen, reduces the residual thrombus burden as assessed via matched optimal coherence tomography examinations.
Methods
Ethics committee approval was obtained from the Ethics Committee of the University of Ferrara on May 30th 2013. In October 2013 the protocol was registered at ClinicalTrials.gov with the identifier number NCT01971788.
PATIENT SELECTION
The MATRIX study is a multicentre, prospective, randomised, open-label, two by three factorial comparison of transradial vs. transfemoral intervention and bivalirudin vs. unfractionated heparin with provisional use of GPI, in patients affected by either non-ST-elevation acute coronary syndrome (NSTE-ACS) or STEMI. Patients randomly assigned to receive bivalirudin will be randomised to stop or to prolong bivalirudin infusion at the end of PCI.
Selection of patients for the MATRIX OCT substudy will be carried out according to the following criteria. 1) Patients referred to the cathlab with an ST-elevation myocardial infarction (STEMI) and matching the criteria for the MATRIX study will be selected for the OCT substudy. 2) Randomisation will be carried out as per the MATRIX protocol, and only patients randomised to bivalirudin treatment will be included in the OCT substudy, whereas patients randomised to heparin±GPI will be part of a registry. 3) After coronary angiography, only patients showing at least one critical stenosis with clinical indication to perform a staged PCI before hospital discharge will finally be enrolled in the study.
Patients with non-ST-elevation acute coronary syndrome will not be included in the OCT substudy, and all the remaining exclusion criteria of the MATRIX study will be applied (Table 1).
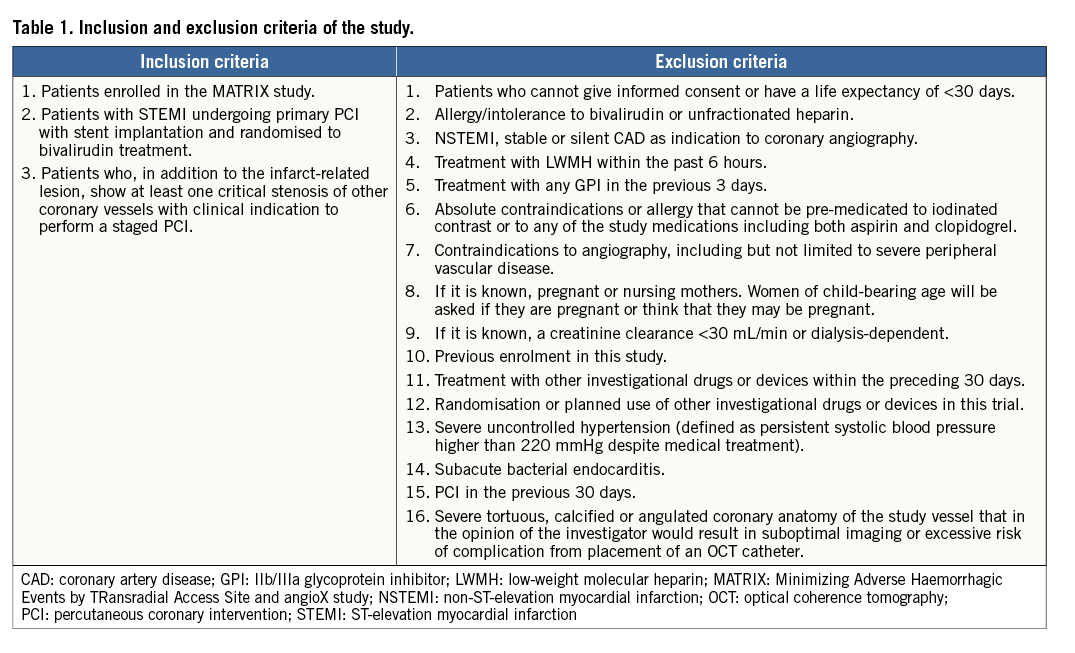
STEMI will be defined as: 1) chest pain for >20 min with an electrocardiographic ST-segment elevation ≥1 mm in two or more contiguous electrocardiogram leads, or with a new left bundle branch block or an inferolateral myocardial infarction (MI) with ST-segment depression of ≥1 mm in ≥two leads V1-V3 with a positive terminal T wave, and 2) admission either within 12 hours of symptoms onset or between 12 and 24 hours after onset with evidence of continuing ischaemia or previous lytic treatment.
STUDY DESIGN
In patients affected by STEMI and multivessel disease, treated by PCI and randomised to either short or prolonged bivalirudin infusion, an OCT evaluation of the infarct-related artery will be carried out at two time points: 1) at the end of primary PCI, and 2) at the time of staged PCI, which will be scheduled three to five days after primary PCI (Figure 1).
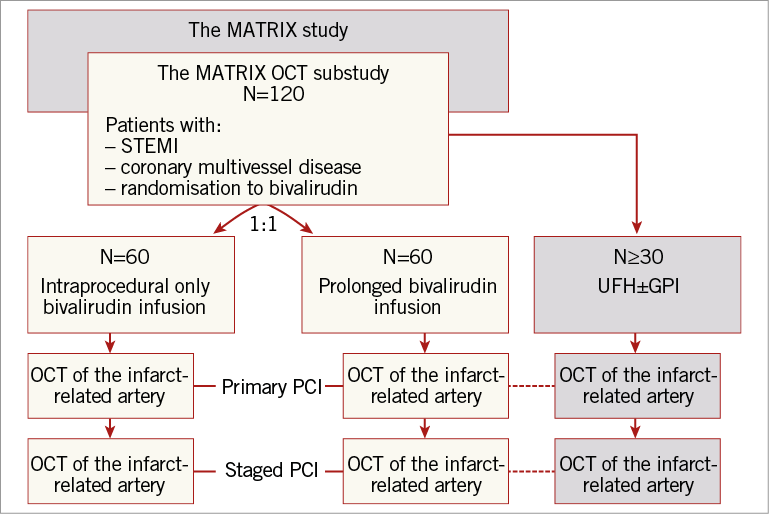
Figure 1. Flow chart of the MATRIX OCT substudy, showing the enrolment procedure. MATRIX: Minimizing Adverse Haemorrhagic Events by TRansradial Access Site and angioX study; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction
Patients with STEMI and multivessel disease randomised to heparin±GPI for whom an OCT evaluation is required due to either angiographic or clinical reasons will be included in a separate registry.
TREATMENT OF SUBJECTS
Bivalirudin will be given upon enrolment as a bolus of 0.75 mg/kg followed immediately by an infusion of 1.75 mg/kg/hr. This infusion will either be stopped upon removal of the angioplasty guidewire in the short infusion arm, or will run continuously at the same dose of 1.75 mg/kg/hr for at least four hours after the end of PCI in the prolonged infusion arm.
According to the main MATRIX protocol, patients will only be eligible for “bail-out” GPI administration. Use of an abciximab bolus+12-hour infusion or an eptifibatide double bolus+12 to 18-hour infusion or a tirofiban 25 μg/kg bolus followed by an 18 to 24-hour infusion will be allowed, during PCI, for the following two reasons only: 1) the presence of a “giant” thrombus adjacent to the stent or in the coronary vessel (length >2x that of the diameter of the coronary vessel) after PCI in the absence of a mechanical obstruction; 2) sustained no-reflow (TIMI 0-1 flow in the absence of a mechanical obstruction, refractory to intracoronary nitrates, adenosine or a calcium channel blocker delivered intracoronary to the distal coronary bed via an infusion catheter). The use of manual or mechanical thrombectomy devices is allowed and will be left to the discretion of the treating physician.
ENDPOINTS
The primary endpoint is defined as the percentage difference in minimal flow area (MinFA) measured at the end of primary PCI and at the time of staged PCI.
MinFA is defined as: (stent area+incomplete stent apposition [ISA] area) – (intraluminal defect+tissue prolapse area).
The secondary endpoint is defined as the percentage difference in the number of stent cross-sections with a thrombotic area >5% at the end of primary PCI and at the time of staged PCI.
OCT EVALUATION
OCT of the infarct-related artery will be carried out at the end of primary PCI and repeated at the time of staged PCI. All OCT examinations will be performed using full anticoagulation with bivalirudin. The catheter will be advanced into the vessel so that the proximal marker is at least 1 cm downstream from the distal edge of the stent. The coronary artery will be flushed during the automated OCT pullback with X-ray contrast at 37 degrees Celsius and a flush rate of 3 ml/sec for the right coronary artery and 4 ml/sec for the left coronary artery. The OCT pullback will be carried out at a speed of 20 mm/sec. At follow-up, the flush settings as applied during the baseline imaging procedure will be repeated.
Analysis of OCT images will be performed by Cardialysis BV (Rotterdam, The Netherlands).
STATISTICAL ANALYSIS
We plan to enrol at least 120 patients (2×60 patients), taking into account a drop-out rate of at least 10%. In the TROFY study, where OCT analysis was performed after stenting in STEMI patients, minimal flow area was 7.08±2.14 mm2 in the thrombectomy group and 6.51±1.99 mm2 in the group without thrombus aspiration15. Since it is anticipated that not all MATRIX OCT patients will undergo manual thrombectomy, as this is per protocol left to the discretion of the treating physician, we hypothesised a MinFA of 6.8 mm2 in the group allocated to the short regimen bivalirudin infusion. Therefore, a potential treatment effect of 20% on the change in MinFA between prolonged and intraprocedural bivalirudin infusion strategy could be shown at a >90% power and a two-sided α-level of 0.05. In the parallel registry, we also anticipate recruiting at least 30 patients who received standard UFH. Therefore, we anticipate a final sample of at least 150 patients who received OCT matched assessment at the end of index PCI and at follow-up.
Discussion
Bivalirudin has been studied in a series of randomised controlled trials and significantly reduces major and minor bleeding and thrombocytopaenia as compared to heparins across a broad range of patients with coronary artery disease while maintaining ischaemia protection11,12,16,17.
Although the most recently released European Society of Cardiology guidelines attributed a class IB to bivalirudin for use as the sole antithrombotic drug for primary PCI9, some safety issues have emerged after primary PCI. In the HORIZONS-AMI trial, where bivalirudin showed better results than heparin+GPI in terms of hard clinical endpoints reduction, an increase in the acute stent thrombosis rate was found in patients treated with this drug12. In another study, this drug, whose infusion was stopped at the end of the procedure, was found inferior to eptifibatide for procedure-related myocardial infarctions18. More recently, the EUROpean aMbulance Acs angioX (EUROMAX) study has confirmed the existence of a distinct excess of acute stent thrombosis in bivalirudin-treated patients19.
In order to overcome this potential drawback of the drug, a prolonged bivalirudin infusion after the end of PCI has been proposed and tested in recent studies. In a first pilot study, where this strategy was adopted during high-risk PCI of NSTEMI patients, it was compared to a UFH/abciximab or eptifibatide strategy. The prolonged infusion warranted a comparable efficacy in preventing myocardial injury and contemporaneously warranted lower bleedings15. In the PROBI VIRI study, a randomised clinical trial of patients with stable and unstable angina undergoing complex PCI, the four-hour drug infusion after PCI significantly decreased the incidence of periprocedural myocardial infarction (6.8% vs. 17%, p=0.041) without jeopardising the intrinsic safety of bivalirudin13. In the PROBI VIRI 2 study, the prolonged infusion strategy has been evaluated in patients with STEMI treated by primary PCI: the four-hour post-PCI infusion showed an early tissue reperfusion comparable to that achieved with abciximab and an improvement of ST-segment recovery compared to standard bivalirudin treatment14.
There are several reasons why the combined anticoagulant and antiplatelet effect of a prolonged bivalirudin infusion could prove effective in the setting of primary PCI: 1) the risk of an early stent thrombosis is maximum in the first hours after obtaining vessel patency, as shown in the HORIZONS-AMI study; 2) the antiplatelet activity of a 600 mg dose of clopidogrel, when given in the catheter laboratory, is adequate several hours after the completion of PCI, when bivalirudin infusion has been stopped; 3) current STEMI guidelines9 recommend using the new P2Y12 inhibitors, prasugrel and ticagrelor, due to their rapid onset of action which could theoretically minimise the need for a prolonged infusion of bivalirudin. Nevertheless, the FABOLUS PRO study showed that the degree of early platelet inhibition achieved after a 60 mg loading dose of prasugrel is suboptimal at least for the first two hours in STEMI patients undergoing primary PCI20 and, in the more recent RAPID study, both prasugrel and ticagrelor have been shown to provide an effective platelet inhibition two hours after the loading dose in only half of the patients, and at least four hours are required to achieve an effective platelet inhibition in the majority of patients21. Moreover, in the acute phase of cardiovascular illness, conditions such as nausea, vomiting, profound sedation or shock may profoundly impair the oral antiplatelet drug bioavailability22,23.
These data confirm that primary PCI is a complex clinical setting where platelet activation is often abnormally high and where the procedure of stenting of the infarct-related artery is performed without functional evidence of a significant antiplatelet effect. Thus, prolonging the infusion of bivalirudin after the end of PCI could provide a temporary antithrombotic coverage until oral antiplatelet drugs have achieved a clinical effectiveness.
Interestingly, despite the use of prolonged bivalirudin infusion after PCI in the EUROMAX study, a clear and consistent signal towards a higher rate of acute stent thrombosis has been observed19. In EUROMAX, 50% of patients were treated with the new P2Y12 inhibitors ticagrelor or prasugrel, and roughly 80% of patients received the 0.25 bivalirudin regimen, whereas 20% of patients received the full bivalirudin regimen at 1.75. These observations have led to the provisional belief that no new P2Y12 inhibitors or prolonged bivalirudin infusion would help to mitigate the risk of acute stent thrombosis. It is worth emphasising that this excess of acute ST events is driven by two events in the control group versus 12 events in the bivalirudin arm. Hence, there were in excess of 10 events in the bivalirudin group. Considering that overall one patient out of two was still treated with clopidogrel, and that less than one patient in four received the full bivalirudin post-PCI infusion regimen, we believe that it remains premature to conclude on the lack of protection offered by new P2Y12 inhibitors and/or fully-dosed bivalirudin towards the occurrence of acute ST.
In the recent “How effective are antithrombotic therapies in PPCI” (HEAT PPCI) trial24, 1,829 STEMI patients were randomised to either heparin or bivalirudin treatment with selective “bail-out” use of abciximab in both groups. The primary efficacy outcome of all-cause mortality, stroke, reinfarction, or unplanned revascularisation occurred in 8.7% of the bivalirudin group versus 5.7% of the heparin group (p=0.01), with no significant differences between groups in the use of abciximab. It is worth noting that patients randomised to bivalirudin were more likely to develop stent thrombosis (3.4% vs. 0.9%; p=0.001) as compared to the heparin strategy.
Although this was a large and well-designed trial, it was conducted from a single centre and used an open-label design. Furthermore, bivalirudin infusion was stopped at the end of the procedure with no post-PCI infusion.
Limitations
The decision to include a STEMI patient randomised to bivalirudin in the OCT substudy will be made after coronary angiography, only in the presence of a multivessel coronary disease with indication to pre-discharge staged PCI. This strategy may lead to potential selection bias since not all patients randomised to bivalirudin will enter the study: patients with no-reflow at the end of primary PCI would not offer a good quality OCT evaluation and should be ruled out, as well as patients with heavily calcified or tortuous vessels for whom it would be difficult to advance an OCT catheter into the coronary artery.
Conclusions
Residual thrombosis of stent struts may occur at the end of primary PCI and may determine distal embolisation of thrombotic debris and further myocardial damage. Prolonging bivalirudin infusion beyond the end of primary PCI has been demonstrated to improve myocardial reperfusion and reduce periprocedural myocardial necrosis. Therefore, we hypothesised that a strategy of long-term bivalirudin infusion may reduce the amount of thrombotic material on stent struts, as compared to the traditional, intraprocedural only, administration of the drug.
If this hypothesis were to be confirmed, the antithrombotic properties of bivalirudin would prove useful even after the end of primary PCI, and the prolonged-infusion strategy would receive further support. Nevertheless, only the final results of the MATRIX study will reveal whether the long-term infusion may improve clinical outcome.
| Impact on daily practice Patients with STEMI frequently show high thrombus burden at the site of coronary occlusion both before and after coronary stent implantation. The use of manual thrombectomy during intervention has been shown to affect thrombus burden only marginally at the end of PCI. The value of bivalirudin infusion to reduce residual coronary thrombus burden when prolonged up to four hours after primary PCI is unknown. The results of our study will shed light on whether prolonging infusion of a direct thrombin inhibitor after index intervention is an effective and safe measure to reduce residual thrombus burden in patients with STEMI undergoing primary intervention. This study will also investigate at secondary endpoint level whether bivalirudin provides superior efficacy in reducing coronary residual thrombus quantified by OCT as compared to standard UFH alone. |
Funding
The MATRIX study has been funded via research grants from The Medicines Company and Terumo Corporation. This OCT substudy will receive co-funding from St. Jude Medical to allow for a centralised core lab activity.
Conflict of interest statement
M. Valgimigli has received honoraria for lectures/advisory board membership and research grants from Merck, Iroko, Eli Lilly and Medtronic; honoraria for advisory board membership and lectures from The Medicines Company, Daiichi Sankyo, Inc., St. Jude and Abbott Vascular; and lecture fees from Cordis, CID and Terumo. The other authors have no conflicts of interest to declare.

