- optical coherence tomography
- drug-eluting stent
- drug-eluting balloon
- restenosis
Abstract
Background: Safety concerns regarding use of drug-eluting stent systems (DES) are related mostly to late stent thrombosis, which is facilitated by incomplete stent endothelial coverage. Specific information about time course and amount of endothelial strut coverage of different DES is required, in order to further refine the concept of antiplatelet therapy after DES implantation. Optical coherence tomography (OCT) is emerging as a new gold standard for endovascular imaging of stents, atherosclerosis progression, vulnerable plaque, and neointimal proliferation. The aim of this study is a comparative evaluation using OCT of the XIENCE® V everolimus-eluting stent (Abbot Vascular, Santa Clara, CA, USA) on one hand, and the bare metal stent Coroflex Blue® postdilated with the paclitaxel-eluting balloon Sequent Please® (both from B Braun Melsungen AG, Melsungen, Germany) on the other hand, with respect to endothelial coverage and neointimal proliferation.
Methods: Eighty patients scheduled for elective percutaneous coronary intervention (PCI) of a native coronary stenosis suitable for DES implantation and OCT imaging are scheduled to be openly randomised 1:1 to either XIENCE® or Coroflex Blue®/Sequent Please®. The study is conducted prospectively at a university high-volume PCI centre with OCT expertise. Angiographic follow-up and time-domain OCT imaging with motorised pull-back at 1 mm/s are planned six months after study stent implantation in all patients. OCT endpoints are: (1) endothelial coverage, expressed as % of struts without coverage and % of stent length containing non-covered struts, and respectively (2) neointimal proliferation, given as % neointimal volumetric proliferation within the whole stent and also as peak focal % neointimal area proliferation. The study is not powered for clinical endpoints, which are: subacute or late stent thrombosis and need for revascularisation of the stent segment. Given the high number of measurements (15 cross-section images / 1 mm stent length), OCT endpoints are likely to reach significance at the level p <0.05, if the drop-out rate in follow-up does not exceed 20%.
Current status: The study is currently on-going and its termination is scheduled for February 2010. (ClinicalTrials.gov identifier: NCT01056744).
Introduction
A major problem in the management of coronary artery disease (CAD) in the past decades was restenosis after percutaneous coronary interventions (PCI), which has been considerably alleviated by the use of drug-eluting stent systems (DES)1-4. However, DES may also develop late restenosis (>1 year after implantation in at least 5-10% of patient), and did not improve the incidence of new atherothrombotic events5,6. Moreover, serious concerns regarding use of DES raised the occurrence of late stent thrombosis (LST) in up to 1.5% of patients, which can subsequently lead to myocardial infarction and death in a significant number of cases7-10. Since LST is facilitated by delayed stent endothelial coverage, long-term therapy with a P2Y12 receptor antagonist such as clopidogrel is mandatory in addition to aspirin after DES placement. On the other hand, double antiplatelet therapy accounts for higher costs and risk of bleeding. Therefore, more specific information about the time course and the amount of endothelial strut coverage of different DES is required to further refine the concept of antiplatelet therapy after DES implantation.
New devices have been designed to overcome this problem, attempting to limit the exposure of vascular structures to antiproliferative drugs to the needed minimum. Paclitaxel-eluting balloons proved to be effective for treatment of in-stent restenosis11-13, and thus the combination of a drug-eluting balloon with a bare metal stent (BMS) might be a promising trade-off between earlier endothelial coverage and inhibition of restenosis14.
The optical coherence tomography (OCT) is a new imaging method for intravascular use15-18. Compared to intravascular ultrasound (IVUS), it provides a higher spatial resolution of 10-20 micrometer and less artefacts19,20. OCT reveals plaque structure and intimal proliferation within the stented area and around the struts, providing excellent image quality21-25. Therefore, OCT is currently emerging as a new gold standard for endovascular imaging of stents, atherosclerosis progression, atherothrombosis and neointimal proliferation. To date, no OCT studies about endothelial coverage of stents treated with paclitaxel-eluting balloons are available.
Methods
Patients
Patients aged >18 years referred to the Cardiology Division of the University Hospital of Jena for coronary angiography and PCI due to suspected or previously documented CAD have been prospectively screened since June 2009. Eligible for the study are patients with native coronary lesions suitable for stent placement and OCT imaging, who gave written consent for participation in this clinical trial.
Exclusion criteria are:
– general: pregnancy and breast-feeding, comorbidity with an estimated life expectancy of less than 50 % at 1-year, scheduled major surgery in the next six months, expected non-compliance, participation in other clinical studies and patients unable to give informed written consent;
– procedural: acute coronary syndromes and cardiogenic shock, previous subacute or late coronary stent thrombosis, known non-responsiveness or allergy to aspirin or to thienopyridines, allergy against everolimus and related drugs, or against taxol derivatives;
– angiographic exclusion criteria: culprit lesion within the proximal 5 mm of a native coronary artery, lesion in a vessel supplied by a saphenous vein graft, reference luminal diameter >4 mm, or an estimated stent length >25 mm, respectively.
This single-centre investigator-initiated study was approved by the local ethical committee (identification number 2392-10/2008), registered (ClinicalTrials.gov identifier: NCT01056744) and has been conducted according to the principles of the Declaration of Helsinki regarding clinical studies in humans.
Study design and protocol
A schematic representation of the study design is shown in Figure1. After coronary angiography, identification of the qualifying lesion for stenting and OCT is performed using the above mentioned inclusion and exclusion criteria. Presence of multiple lesions scheduled to be treated in one or more PCI sessions is allowed, but only one coronary vessel segment should be chosen for stenting and OCT as study vessel segment. If the angiography has been performed on another occasion within the past four weeks, a decision can be made without performing a new catheterisation. Noninvasive techniques (multislice computer tomography or magnetic resonance imaging) are not accepted for selection of qualifying lesion. Written consent for the study is obligatory in all cases. Patients are 1:1 randomised between a BMS/DEB arm (Coroflex Blue® stent postdilated with a Sequent Please® balloon) and a DES arm (XIENCE® stent) and orally loaded with 100mg aspirin and 300mg clopidogrel one day before PCI. All other cardiovascular drug prescriptions are not influenced by the study. A number of 40 patients per study arm is estimated to be sufficient for the primary OCT endpoint, if the drop-out rate in follow-up does not exceed 20%.
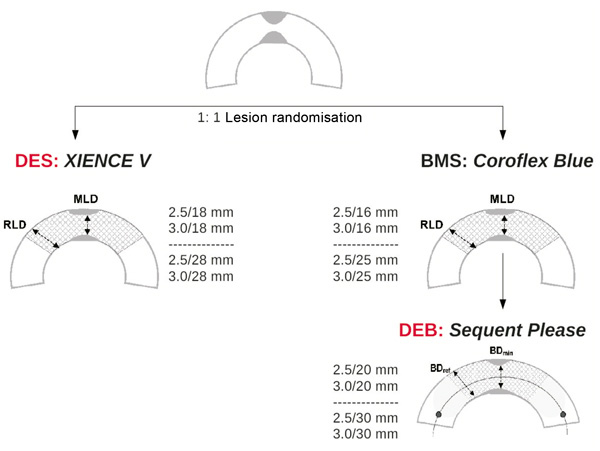
Figure 1. Flow chart showing the design of the OCTOPUS trial.
To increase the homogeneity of the data, only two stent lengths and sizes were accepted in each study arm:
– for DES: 2.5×18, 2.5×28, 3.0×18, and 3.0×28mm XIENCE® stents
– for BMS/DEB: 2.5×16/2.5×20, 2.5×25/2.5×30, 3.0×16/3.0×20, and 3.0×25/3.0×30mm combinations of Coroflex Blue®/Sequent Please®
Index PCI procedure
After assignment to either BMS or DES in an appropriate and per protocol accepted size and length, PCI is performed using 6Fr guiding catheters and heparin to maintain an activated clotting time >250s. Patient, operator and cathlab staff are not blinded to the assigned devices. Single stent use covering the entire lesion should be attempted in the study vessel segment. If necessary, lesions may be predilated before placement of the study stent, which is implanted at a pressure of 12-14atm. In the BMS/DEB arm, stents are postdilated with the assigned DEB, which is carefully placed using stent boost imaging or similar fluoroscopic settings to ensure proper stent coverage and to avoid geographic mismatch. Further postdilations using non-drug-eluting balloons may be performed in both study arms according to the angiographic result at the discretion of the operator. If additional stenting is definitely needed after placement of the study stent, the same stent type (bare metal or drug-eluting) should be used, in a size and length decided by the operator. If additional bare metal stents are placed to cover a persistent dissection, they must not necessarily be postdilated with a DEB. Antithrombotic therapy, sheath management and patient care after the procedure are performed as usual. After PCI all patients give written consent to continue combined antiplatelet therapy with aspirin 100mg/d and clopidogrel 75mg/d for 12 months.
Six-month follow-up with optical coherence tomography
All patients are scheduled for readmission and catheterisation at 6-month follow-up after index PCI. Target vessel should be engaged with a 7Fr guiding catheter via femoral approach. Quantitative coronary angiography (QCA) is performed off-line using the medical imaging software Osiris (University of Geneva, Switzerland). Time-domain OCT of the study stent segment is performed with the M2 CV system (LightLab Imaging Inc., Westford, MA, USA) using the occlusive technique. The standard guidewire is thus exchanged for the OCT image wire using a special over-the-wire low-pressure balloon catheter, which is finally placed just before the proximal stent end, while the tip of the wire has been advanced about 2cm far from the distal stent end. After vessel occlusion with the inflated balloon at 0.7-1 atm, the artery is flushed with saline manually, or using an automatic injector at 1-2ml/s. When OCT image quality is adequate, the motorised pull-back at 1 mm/s is started and digital OCT recording is performed. In the case of longer stents (>20 seconds pull-back) or if signs of ischaemia occur, several shorter pull-backs are performed for the same study stent. After OCT, postprocedural management and sheath removal are performed according to hospital’s standards of care.
Interpretation of optical coherence tomography data
OCT runs are digitally recorded and saved as raw data in proprietary format (LightLab Imaging), and, as well, as compressed AVI files. Raw data are off-line analysed using the available software on the OCT machine by two independent investigators (F.J., J.G.), which are blinded to the demographic, angiographic, and clinical findings. Additionally, AVI files are read and imported by a dedicated research software that enables automatic continuous measurements of cross-sectional areas, strut recognition and classification, and volume calculations, requiring much less amount of user input. Discordances between the two investigators are resolved by consensus reading (T.C.P., S.O.). Analysis of stent cross sections is performed at maximum 1 mm intervals on homogeneously endothelialised segments, or even frame five by frame, if required. OCT analysis was performed to assess: (1) neointimal proliferation, and (2) endothelialisation of the stent struts, dividing the stent length in subsegments: native vessel 2-5mm proximal from stent end, proximal 2-5mm of the stent, medial stent segment, distal 2-5mm of the stent and, in the native vessel, 2-5mm distal from stent end, respectively. Thus, quantitative OCT parameters are reported for each stent subsegment and for the whole stent as well.
Assessment of neointimal proliferation
Quantitative analysis is manually carried out using following parameters (Figure 2):
LA: lumen area
SA: stent area
NIA: neointimal area = SA – LA
EEMA: external elastic membrane area (if measurable)
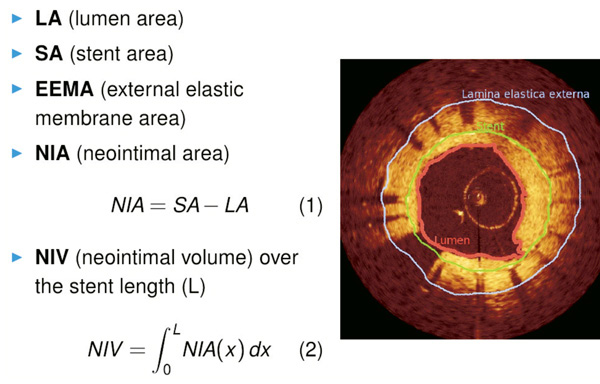
Figure 2. Quantitative assessment of neointimal proliferation on cross-sectional images.
Values obtained at the site of maximal proliferation are selected and stored in the database for each of the five previously defined subsegments, as well as for the entire stent segment. Automatic analysis using the dedicated software is employed to compute the neointimal volume NIV as an integral of NIA over the stent length. To handle the gaps with poor image quality or artifacts that are excluded from the analysis, NIV is finally expressed as a normalised value to 10mm stent length.
Additionally, the pattern of proliferation –if any– is qualitatively assessed in each subsegment and classified upon previously described criteria23:
– neointimal structure: homogeneous, heterogeneous or layered
– neointimal backscatter: high or low intensity
– lumen shape: regular or irregular
– intraluminal material: present or absent
– microvessels: present or none, and if any, the number and maximal diameter
Assessment of endothelial coverage of stent struts
Analysis of stent strut endothelialisation is performed for each stent segment, both manually and using the dedicated software. The algorithm used for strut classification is schematically given in Figure3 and illustrated by examples (Figure 4). Thus, stents struts are classified as:
– contained within the vessel wall (distance between strut surface and the lumen-wall border >0.1 mm)
– apposed (situated < 0.1 mm from the lumen-wall border). These struts can be:
• covered by endothelium
• uncovered
– floating (in the lumen, >0.1 mm away from the vessel wall). They can also be:
• covered by endothelium (possibly due to late vessel remodelling)
• uncovered (possibly due to stent underexpansion)
• as a particular case: over the take-off of a side branch
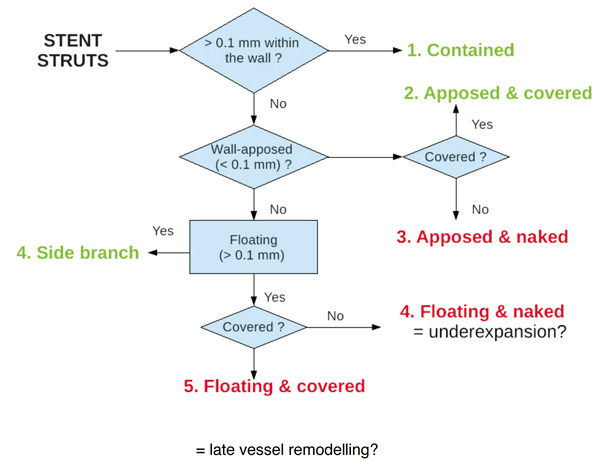
Figure 3. Schematic description of the proposed algorithm for stent strut classification.
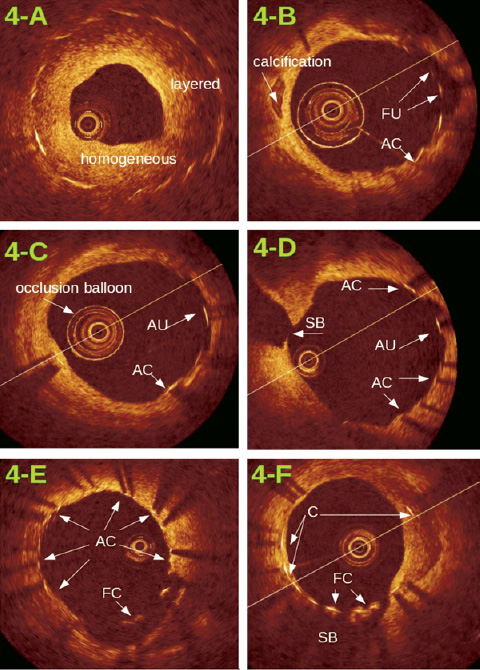
Figure 4. Examples of proliferation patterns and stent strut classification: 4-A: significant proliferation, all struts are contained in the wall, proliferation pattern varies from homogeneous to layered. 4-B – 4-F: very limited proliferation, typical for drug-eluting devices. Stent struts are classified as: contained in the wall (C), apposed and covered (AC), apposed but uncovered (AU), floating and uncovered (FU), and floating but covered (FC). Images 4-D and 4-F also show the take-off of a side branch (SB) covered by stent struts.
Endpoints
The primary OCT endpoint is the endothelial stent coverage at 6-months, defined firstly as % of uncovered struts, and secondly as % of interpretable frames showing uncovered struts.
The secondary OCT endpoint is the neointimal proliferation at 6-months in the study stent, defined regionally as the highest value for neointimal area (NIA) within the stent, and also globally as neointimal volume (NIV) normalised for 10mm stent length.
The study is not powered for clinical endpoints, which are: acute, subacute or late thrombosis of the study stent according to the ARC definition26,27, and the need for revascularisation of the study vessel segment within 6-months. Furthermore, occurrence of major adverse cardiac events (cardiac death, myocardial infarction, need for revascularisation) will be assessed for safety reasons at 6-, 12- and 24-months by telephone interview.
Laboratory studies
Blood samples are obtained before index PCI and at follow-up. The number of circulating endothelial progenitor cells is assessed by several epitopes detected using fluorescent activated cell sorter analysis. Furthermore, the circulating levels of different inflammatory cytokines and endothelial micro-particles are determined in addition to the routinely measured parameters of lipid metabolism.
Data management and statistical analysis
Demographic, clinical, angiographic and procedural data, as well as the OCT parameter and laboratory data are archived into a custom-made Microsoft Access (Microsoft Inc, Redmond, WA, USA) database, whose forms serve as electronic case-report forms for data input.
Data analysis is performed using the statistical package SPSS v.17 (SPSS Inc., Chicago, IL, USA), accepting p-values <0.05 as significant. All continuous variables are expressed as mean values ±SD. Multiple comparisons between different device groups are performed for numeric variables by one-way analysis of variance using Bonferroni’s and Tamhane’s T2 post hoc tests. Categorical variables are compared by Pearson’s 2test. Receiver operator characteristic (ROC) analysis is used to find predictors for neointimal proliferation and endothelial coverage of stent struts.
Discussion
To date only a few clinical studies employed the unique capabilities of OCT as high-resolution real-time imaging modality to describe the time course of stent endothelialisation in humans21-25. Furthermore, to the best of our knowledge, no OCT data after combined BMS/DEB therapy are currently available. The present OCTOPUS trial is intended to systematically describe the patterns of tissue growth and to quantify the exact amount of neointimal proliferation after two different drug-delivery-based endovascular therapies. In case of very limited proliferation in the stent –which is actually expected in both study arms– a comprehensive analysis of the endothelial coverage of the struts is carried out using a novel algorithm for strut classification. Finally, a dedicated software for three-dimensional reconstruction and image classification is expected to enable a complete stent segment analysis and to limit variability of observations. Currently, despite the enormous potential of OCT for automatic stent and plaque recognition, only very few data are available on this topic28.
From a clinical point of view, the study tests the hypothesis, whether the post-dilation with DEB after BMS implantation can provide better results than the recent PEPCAD III study, where the premounted BMS on a DEB was inferior to sirolimus-eluting stents. Theoretically, due to the longer DEB, in this study no increased restenosis at the stent edges should be expected.
Finally, clinical results at mid- and long-term after intravascular therapy with drug-eluting stents or balloons depend not only on the device itself and the lesion characteristics, but probably also on the capacity of the circulating endothelial progenitor cells to dock and rapidly accomplish the endothelial coverage. Additionally, the inflammatory background and plaque composition are responsible for periprocedural microembolisation and may also trigger restenosis. The laboratory studies from the OCTOPUS trial are seeking out the missing link between the patient’s ability for endothelial regeneration, the vascular inflammation and the long-term response to stent placement.
Study limitations
Several methodological aspects need to be addressed. With respect to the patient population, the sample size is relatively low due to the single-centre study design, and therefore this trial cannot be powered for clinical endpoints. The use of different stent and balloon lengths leading to smaller subgroups will further challenge the statistical analysis. Regarding the OCT technology; time-domain imaging has been employed, not the modern frequency-domain OCT which provides superior temporal and spatial resolution. Another expected limitation is related to the interpretation of OCT runs, where the accuracy of volumetric analysis is hampered when a stent cannot be imaged within a single pullback, and also if gaps with poor quality are encountered. However, due to the systematic measurement approach, the results are still likely to be valid and reproducible.
Clinical implications
Clopidogrel is currently recommended for at least 12 months after DES implantation to avoid late stent thrombosis based on a significant body of evidence from randomised trials and registry data9,10,26,27. With the wider use of DES during elective and acute PCI on one hand and the increased population age on the other hand, the raising iatrogenic bleeding risk seems to be traded off against lower stent restenosis. This aspect is particularly important in patients on chronic oral anticoagulation, who are definitely at high risk for bleeding during a 12-month triple antithrombotic therapy. OCT has the potential to exactly assess the stent apposition and endothelial coverage, which is directly related to the need for combined antiplatelet therapy. Therefore, use of OCT at follow-up after stent placement has the potential to refine the clinical decision to stop or continue clopidogrel treatment, which can be mandatory in case of bleeding complications or elective surgery.
Conflict of interest statement
Dr. Poerner received research grants from Abbott Vascular, B Braun and Boston Scientific in addition to local institutional funding. The other authors have no conflicts of interest to disclose with respect to this study.
References

