
Abstract
Aims: Randomised trials have demonstrated improvement in clinical outcomes with intravascular ultrasound (IVUS)-guided percutaneous coronary intervention (PCI) compared with angiography-guided PCI. The ILUMIEN III trial demonstrated non-inferiority of an optical coherence tomography (OCT)- versus IVUS-guided PCI strategy in achieving similar post-PCI lumen dimensions. ILUMIEN IV is a large-scale, multicentre, randomised trial designed to demonstrate the superiority of OCT- versus angiography-guided stent implantation in patients with high-risk clinical characteristics (diabetes) and/or complex angiographic lesions in achieving larger post-PCI lumen dimensions and improving clinical outcomes.
Methods and results: ILUMIEN IV is a prospective, single-blind clinical investigation that will randomise between 2,490 and 3,656 patients using an adaptive design to OCT-guided versus angiography-guided coronary stent implantation in a 1:1 ratio. The primary endpoints are: (1) post-PCI minimal stent area assessed by OCT in each randomised arm, and (2) target vessel failure, the composite of cardiac death, target vessel myocardial infarction, or ischaemia-driven target vessel revascularisation. Clinical follow-up will continue for up to two years. The trial is currently enrolling, and the principal results are expected in 2022.
Conclusions: The large-scale ILUMIEN IV randomised controlled trial will evaluate the effectiveness of OCT-guided versus angiography-guided PCI in improving post-PCI lumen dimensions and clinical outcomes in patients with diabetes and/or with complex coronary lesions. Trial registration: NCT03507777
Introduction
Angiography, the most commonly used imaging modality to guide percutaneous coronary intervention (PCI), has several known limitations, including imprecision in determining plaque morphology, vascular remodelling, and atherosclerosis burden1,2. It is suboptimal in identifying stent underexpansion, malapposition, thrombus, residual dissection, and plaque protrusion3. These limitations can partly be overcome by intravascular ultrasound (IVUS)1,2, which allows cross-sectional tomographic imaging of the vessel wall. Findings from large observational cohort studies, randomised trials, and meta-analyses have shown that, by achieving larger luminal dimensions compared with angiography guidance, IVUS-guided drug-eluting stent (DES) implantation reduces major adverse cardiovascular events, including target lesion revascularisation, stent thrombosis and cardiac mortality4,5,6,7,8. Despite these results and guideline recommendations9, IVUS-guided PCI remains infrequently used. Difficulty in image interpretation due to relatively low axial resolution (50-200 μm), poor discrimination of plaque subtypes, additional procedural time and incremental cost are often cited as principal reasons for low IVUS adoption rates.
Optical coherence tomography (OCT) is a newer intravascular imaging modality that provides rapid acquisition of higher resolution images (10-20 μm) compared with IVUS, thus allowing more accurate identification of thrombus, lipid, calcium, fibrous cap thickness, dissections, plaque prolapse, stent malapposition, and strut coverage (although tissue penetration depth is lower with OCT than with IVUS)10. OCT also measures luminal and stent dimensions more accurately than IVUS11. Nonetheless, few studies of OCT-guided stenting have been performed10,12. In the ILUMIEN III randomised controlled trial, in patients with non-complex lesions, an OCT-specific stent sizing and optimisation strategy was safe and non-inferior to IVUS and angiography guidance with respect to post-PCI luminal dimension, the primary endpoint of the trial. Moreover, OCT was superior in achieving larger stent expansion compared with angiography3. The ILUMIEN IV trial was thus designed to assess whether this OCT-guided PCI strategy would result in improved clinical outcomes compared with angiographic guidance, driven by an increased minimal stent area (MSA).
Methods
OBJECTIVE AND STUDY DESIGN
ILUMIEN IV is a prospective, single-blind randomised controlled trial assigning subjects to OCT- versus angiography-guided coronary stent implantation in a 1:1 ratio (Figure 1). The objective of the trial is to demonstrate the superiority of OCT-guided PCI in (a) achieving larger acute post-PCI lumen dimensions, and (b) improving cardiovascular outcomes in patients with diabetes and/or complex lesions. The clinical investigation is being conducted at approximately 100 centres in North America (USA and Canada), Europe, Middle East, and Asia-Pacific. Up to 3,656 randomised patients and approximately 375 roll-in patients will be enrolled. The expected duration of patient recruitment is approximately two years and all enrolled patients will be followed for two years. The total duration of the study is expected to be five years. The trial was designed by the principal investigators, steering committee, and sponsor (Abbott, Santa Clara, CA, USA) and is registered at ClinicalTrials.gov (unique identifier: NCT03507777). The trial is funded by Abbott. Since the initiation of the study in March 2018, one formal protocol amendment has been submitted and approved by the US FDA.
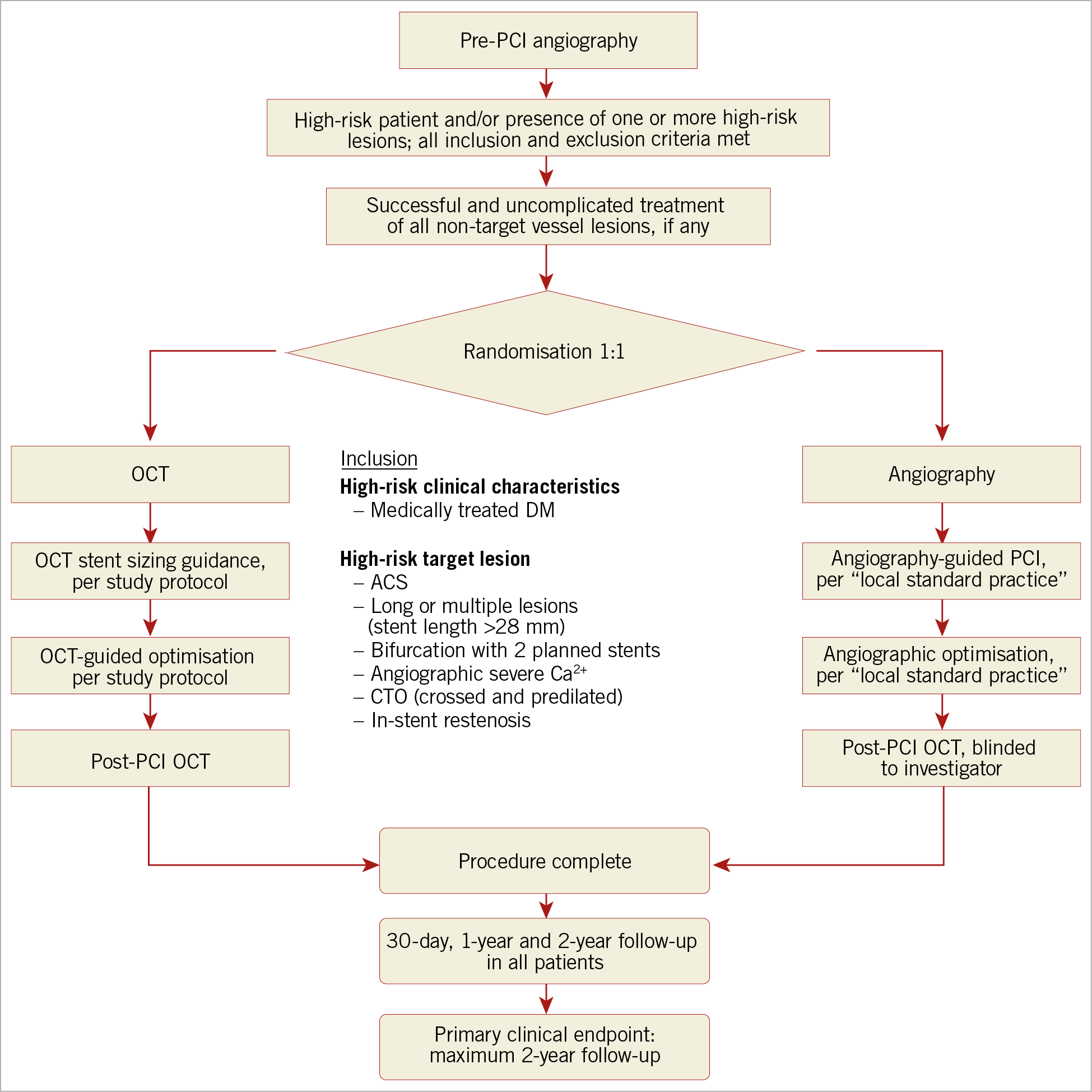
Figure 1. Patient flow for screening, randomisation, and follow-up in the ILUMIEN IV trial. OCT: optical coherence tomography; PCI: percutaneous coronary intervention
STUDY POPULATION, INCLUSION AND EXCLUSION CRITERIA
The key general and angiographic inclusion/exclusion criteria for enrolment in the ILUMIEN IV trial are shown in Supplementary Table 1. Briefly, using an adaptive design, between 2,490 and 3,656 patients undergoing clinically indicated PCI with XIENCE everolimus-eluting stents (Abbott Vascular, Santa Clara, CA, USA) will be enrolled if they have either (a) high-risk clinical features, defined as medication-treated diabetes mellitus, and/or (b) one or more complex lesions, defined as:
i. A target lesion responsible for either
– Non–ST-segment myocardial infarction, defined as a clinical syndrome consistent with an acute coronary syndrome and a minimum troponin of 1 ng/dL (may or may not have returned to normal), or
– ST-segment myocardial infarction >24 hours from the onset of ischaemic symptoms
ii. Long or multiple lesions, defined as intended total stent length (continuous or separated) in any single target vessel ≥28 mm.
iii. A bifurcation lesion intended for treatment with a stent in both the main branch and side branch wherein the side branch stent is ≥2.5 mm in diameter by angiographic visual estimation.
iv. Angiographic severe calcification, defined as visible calcification on both sides of the vessel wall in the absence of cardiac motion.
v. A chronic total occlusion; randomisation is permitted only after successful crossing with antegrade wire escalation and predilatation.
vi. Diffuse or multifocal pattern in-stent restenosis with lesion at or within the existing stent margin(s).
The principal rationale for inclusion of complex target lesions and/or diabetic patients in the ILUMIEN IV trial was to include a study population in whom the event rate after contemporary DES implantation is still suboptimal despite angiographic guidance. Identification of the candidate target lesions and clinical risk characteristics was based on an analysis of pooled individual patient randomised controlled trial and registry data13,14 (Supplementary Table 2), as described in Supplementary Appendix 1. Patients with advanced chronic kidney disease (creatinine clearance ≤30 ml/min/1.73 m2) and not on dialysis are excluded due to the risk of contrast-induced nephropathy; however, patients with end-stage renal disease on dialysis are eligible for enrolment. Patients with ST-segment myocardial infarction within 24 hours of symptom onset are excluded because of the relatively high rates of non-analysable pre-PCI OCT acquisitions due to high thrombus burden, inefficacy of thrombectomy to reduce the thrombus burden, and poor blood clearance15. Of note, very long lesions, multiple complex coronary lesions, including in-stent restenosis, bifurcation lesions, and chronic total occlusions were excluded from the ILUMIEN III trial. The superior resolution of OCT would be expected to be of greater clinical impact in this complex subset of lesions in which the risk of stent failure is higher than in non-complex lesions, especially in high-risk patient cohorts such as diabetics16. Thus, the principal hypothesis of the ILUMIEN IV trial is that, in patients with diabetes and/or complex coronary lesions, the better morphologic lesion characterisation, superior procedural planning, and enhanced DES optimisation (correcting suboptimal results and major procedural complications) afforded by OCT compared with angiography will result in improved acute procedural results and superior long-term clinical outcomes.
In addition to the inclusion criteria of diabetic patients and/or complex lesion characteristics, target lesions must have a visually estimated or quantitatively assessed diameter stenosis of ≥70% or diameter stenosis ≥50% plus non-invasive or invasive evidence of ischaemia or haemodynamic significance, or be deemed to be the culprit lesion responsible for a biomarker-positive acute coronary syndrome (e.g., presence of plaque disruption or thrombus). Based on visual estimation on pre-PCI angiography, the estimated stent diameters must be ≥2.5 mm and ≤3.5 mm. This criterion is mandated in order to comply with the US FDA instructions for the use of coronary OCT systems, and to exclude stent implantation in very small target vessels that are frequently located at the distal ends of the arteries where the rapid-exchange length of the OCT catheter may preclude imaging of diseased segments. In addition, these criteria exclude stent implantation in very large target vessels for which stent failure rates are recognised to be relatively low with angiographic guidance alone13. Nonetheless, if the measurements on OCT are outside the visually estimated angiographic diameter range, stent sizing and post-dilatation will be based on the OCT measurements, following the detailed study protocol. Up to two target lesions requiring PCI may be present in any single target vessel, with a maximum of two target vessels in one subject allowed for randomisation. Thus, up to four randomised target lesions per patient in a maximum of two target vessels, including major side branches, may be included. The intended target lesions will be declared prior to randomisation. Complex multivessel coronary disease with a SYNTAX score ≥33 is excluded unless the Heart Team, including a cardiac surgeon, concludes that PCI is appropriate (e.g., the surgical risk is too high). Left main coronary target lesions are excluded.
PRIMARY ENDPOINTS AND SAMPLE SIZE CALCULATION
There are two separately powered co-primary endpoints, both of which must be met to declare trial success (Supplementary Table 3). (1) The imaging endpoint is the final post-PCI MSA per target lesion assessed by OCT (a blinded OCT run will be performed in the angiography-guided arm after all interventions) by an independent imaging core laboratory masked to treatment allocations. (2) The clinical co-primary endpoint is target vessel failure (TVF), defined as the composite of cardiac death, target vessel myocardial infarction, or ischaemia-driven target vessel revascularisation, assessed at up to two years (Supplementary Table 3). Clinical events will be monitored on-site, and an independent clinical events committee will adjudicate all events after review of original source documents, blinded to treatment assignment.
The imaging hypothesis of the study is that OCT guidance is superior to angiography guidance in achieving a larger final MSA. The post-PCI MSA has repeatedly been shown to be the strongest and most consistent stent-related parameter to predict DES clinical outcomes17,18,19,20,21,22,23,24,25. To detect a minimum difference in MSA of 0.4 mm2 with a standard deviation of 2.2 mm2, 1,600 subjects randomised 1:1 to OCT versus angiography will provide 95% power to detect superiority with a one-sided α of 0.025. After 1,600 subjects are enrolled, the primary endpoint of MSA will be internally assessed between the groups by a blinded committee. If a larger MSA in the OCT-guided arm is demonstrated, the trial will continue enrolling subjects to assess the clinical co-primary endpoint. Otherwise, the trial may be terminated for futility. The final MSA endpoint will be tested and reported in all subjects in whom a stent was implanted.
The clinical hypothesis is that OCT guidance is superior to angiography guidance with respect to TVF rates at two years. The sample size is based on an assumption of cumulative TVF rates in the angiography-guided arm at one and two years of 8.0% and 12.0%, respectively. The basis for these rates is provided in Supplementary Appendix 2, Supplementary Table 4 and Supplementary Table 5. To detect a 35% reduction in hazard with OCT guidance, assuming a 5% rate of loss to follow-up each year, 2,490 subjects randomised 1:1 to OCT guidance versus angiography guidance would provide 85% power to demonstrate superiority with a one-sided α of 0.025. When 50% of the anticipated TVF events have occurred (n=194), the data safety monitoring board will decide whether sample size adjustment is needed. If the interim analysis indicates the need for a sample size increase, enrolment will be adjusted up to a total of 3,656 subjects in an adaptive design26.
PRE-SPECIFIED SECONDARY ENDPOINTS
If both primary imaging and clinical endpoints are met, numerous secondary endpoints will be examined, categorised as: i) procedural measures; ii) angiographic measures (based on core laboratory quantitative coronary angiography); iii) OCT-defined measures (based on core laboratory quantitative OCT assessments); iv) clinical outcomes (assessed at 30 days, one year, and two years); v) patient-reported outcomes; and vi) costs and cost-effectiveness analyses. A detailed description of the secondary endpoints is provided in Supplementary Table 3.
OCT IMAGE ACQUISITION AND STENT OPTIMISATION PROTOCOL
Intravascular OCT is performed using a commercially available system (the ILUMIEN™ OPTIS™, OPTIS Integrated, and OPTIS Mobile systems; Abbott Vascular) that incorporates a rapid exchange catheter (Dragonfly™ DUO, Dragonfly™ OPTIS™, Dragonfly™ OpStar™ Imaging Catheter; Abbott Vascular) and an integrated pullback system (18-36 mm/s), acquiring images at high (~15 μm) axial resolution with blood displacement. Images are acquired after predilation, if necessary, and after administration of intracoronary nitroglycerine. The automated OCT-angiography co-registration (where available) will be used to guide PCI in the OCT arm of the study according to the stent sizing and optimisation algorithm, slightly modified from the methodology described in the ILUMIEN III trial3. The algorithm includes measurement of the external elastic lamina (EEL)-based vessel diameter in the proximal and distal reference segments, and has been designed to achieve larger stent dimensions and more complete lesion coverage than would occur with sizing to the proximal and distal reference lumens3. An overview of the OCT-guided stent optimisation algorithm and details for post-implant stent optimisation are summarised in Figure 2. A step-by-step description of OCT image acquisition to guide procedure planning and decision making together with several representative examples are provided in Supplementary Appendix 3 and Supplementary Figure 1-Supplementary Figure 6.
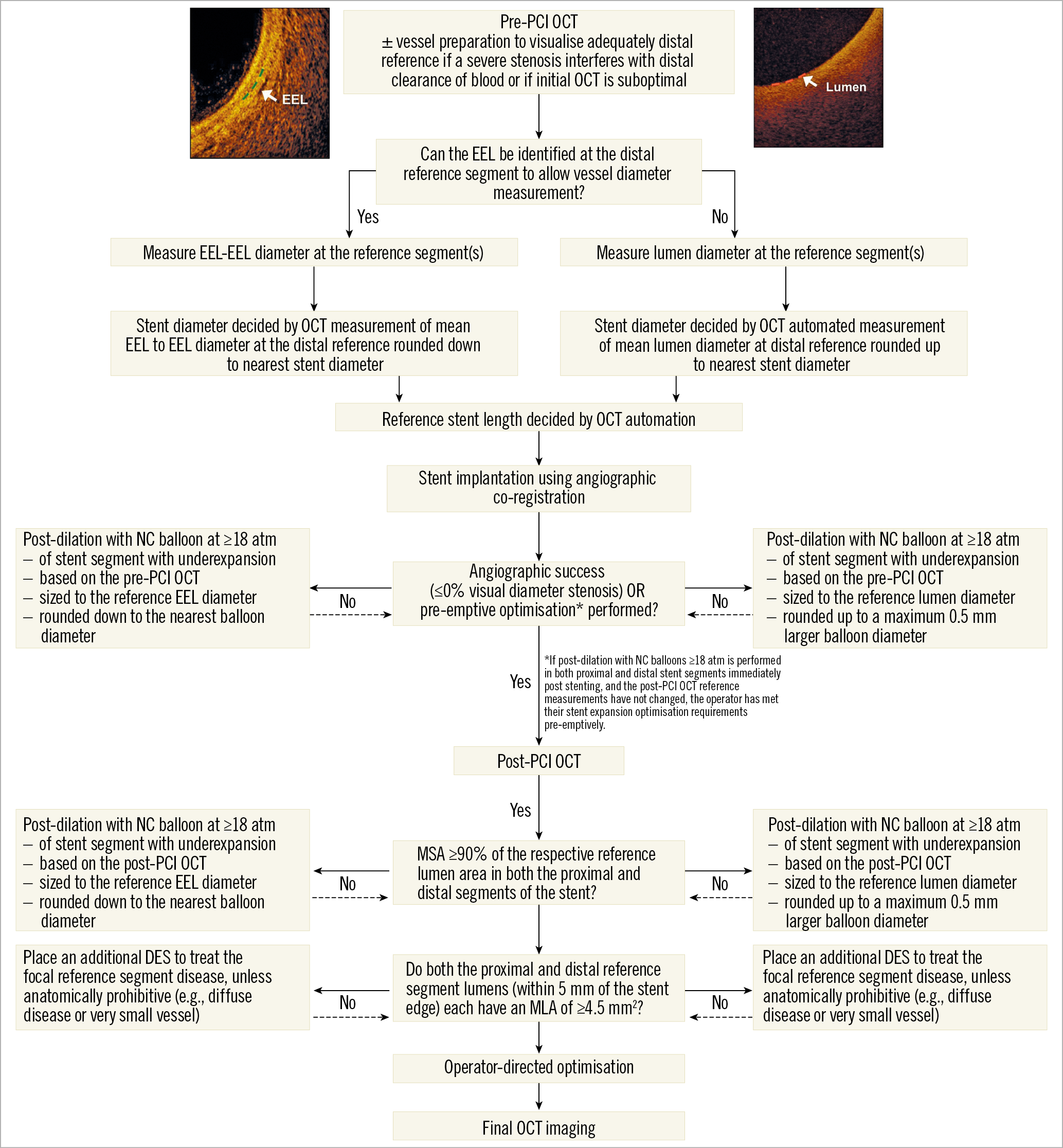
Figure 2. The algorithm for OCT-guided PCI optimisation in ILUMIEN IV. OCT-guided assessment pre-PCI through stent implantation. Vessel diameter must be assessed as the EEL-EEL diameter at the reference segments, unless the EEL cannot be identified, in which case luminal measures are used. OCT-guided optimisation post stent implantation is described per EEL-based diameter measurement and per lumen-based diameter measurement. EEL: external elastic lamina; MLA: minimal lumen area; MSA: minimal stent area; NC: non-compliant; OCT: optical coherence tomography
In brief, the proximal and distal reference mean EEL-based diameters will be measured, and the distal reference EEL diameter will be rounded down to the nearest available stent size (usually in 0.25 mm increments) to determine stent diameter. If the EEL cannot be adequately visualised, the stent diameter is chosen using the mean lumen diameter at the distal reference rounded up to the next stent size. Stent length will be determined by the distance from distal to proximal reference site using the OCT automated lumen detection feature. After stent deployment, optimisation will be performed with non-compliant balloons in the proximal and distal segments of the stent based on the respective EEL or lumen diameter measurements by rounding down (EEL) or up (lumen) to the nearest non-compliant balloon size. Following optimisation, OCT imaging will be repeated and, if necessary, iterative high-pressure or larger non-compliant balloon inflations performed, based on new reference segment measurements, in an attempt to achieve acceptable stent expansion (an MSA of at least 90% in both the proximal and distal segments of the stent relative to the closest reference segment). Following OCT-guided stent expansion optimisation, the proximal and distal reference segments, defined as 5 mm from the edges of the stent, are examined for inflow/outflow disease. If both the proximal and distal reference segments have a minimal lumen area ≥4.5 mm2, no further treatment is necessary. If there is untreated reference segment disease defined as a focal minimal lumen area <4.5 mm2 in either proximal or distal reference segments following the additional OCT run, an additional DES must be implanted unless anatomically prohibitive (e.g., biological vessel tapering, distal diffuse disease, absence of landing zone). If there is a major edge dissection, defined as ≥60° of the circumference of the vessel at the site of dissection and ≥3 mm in length, it is recommended that additional DES be placed to correct the abnormality unless anatomically prohibitive.
To ensure that the participating sites follow the detailed study protocol, rigorous training was provided during the preparatory and roll-in phases of the trial. The investigators performed online training in which they were required to interpret images and perform measurements based on the study protocol. Investigators also attended in-person training conducted by the principal investigators or a sponsor representative trained directly by the principal investigators and were required to pass an in-person examination using the commercial OCT software prior to enrolment. Additionally, an online system for monitoring protocol compliance with the OCT-guidance procedures is utilised whereby the OCT core laboratory rapidly assesses the images received and sends a report back to the study sites within 72 hours to provide feedback. The report includes the core laboratory OCT measurements and determination of any minor or major protocol violations. If major protocol violations were present, the operator will be asked to stop enrolling participants until they receive further training. If an operator accrues three major protocol violations, they may be asked to withdraw from the study although their previously enrolled randomised patients will remain in the study for intention-to-treat analysis.
DATA COLLECTION AND ANALYSIS
Quantitative analyses of the data will be performed at OCT and angiography core laboratories (Cardiovascular Research Foundation, New York, NY, USA). All primary and secondary endpoints and their relationship to OCT use or the PCI procedure are adjudicated to pre-specified definitions by an independent clinical events committee (Cardiovascular Research Foundation) after review of original source documents, except for site-reported secondary endpoints specified in Supplementary Table 3. Data management and study analyses are performed by the sponsor. Details of the planned statistical analyses appear in Supplementary Appendix 4. The primary analysis will be intention-to-treat, but a separate per protocol analysis will be performed as a sensitivity analysis. Missing data will be left missing. Imputation for the co-primary endpoints of MSA or TVF will not be performed. The principal investigators and chairman have complete access to the database and accept responsibility for the design and conduct of the study, all study analyses, and the drafting and editing of the principal and subsequent reports.
Discussion
By providing detailed visualisation of vessel architecture and accurate measurement of vessel dimensions, OCT offers the potential to improve acute PCI results and subsequent clinical outcomes compared to the standard angiography guidance. The ILUMIEN IV trial is designed to establish superiority of OCT-guided PCI in achieving larger luminal dimensions post PCI and clinical superiority of the OCT guidance strategy in lowering TVF in patients with diabetes and/or complex lesions treated with contemporary DES.
Limitations
Since this is a trial design manuscript, there are no limitations to specify at this time.
Conclusions
The trial is currently recruiting, with 1,650 patients enrolled at the time of manuscript submission. The principal results of ILUMIEN IV are expected in 2022.
|
Impact on daily practice ILUMIEN IV is a large randomised trial that will determine whether OCT guidance will improve post-PCI luminal dimensions and clinical outcomes compared with the standard angiography guidance in patients with diabetes and/or complex coronary lesions. |
Funding
ILUMIEN IV is funded by Abbott (Santa Clara, CA, USA).
Conflict of interest statement
Z.A. Ali reports institutional research grants to Columbia University and Cardiovascular Research Foundation from Abbott and Cardiovascular Systems Inc., being a consultant for Abbott, Amgen, AstraZeneca, Abiomed, Boston Scientific, Cardinal Health, Opsens Medical, and ACIST Medical, holding equity in Shockwave Medical, and receiving grants from the National Heart, Lung and Blood Institute. U. Landmesser reports being a consultant for Abbott, Boston Scientific, and Biotronik. A. Maehara reports grant support from Abbott Vascular and Boston Scientific, and being a consultant for Conavi Medical Inc. M. Matsumura reports being a consultant for Terumo Corporation. G. Guagliumi reports institutional grant support from Abbott Vascular, Boston Scientific, Infraredx, and St. Jude Medical, and being a consultant for Abbott Vascular, Boston Scientific, and St. Jude Medical. M.J. Price reports consulting fees and speaker’s honoraria from Abbott Vascular, AstraZeneca, Boston Scientific, Chiesi USA, and Medtronic, consulting fees from W.L. Gore & Associates, and ACIST Medical, and grants (to the institution) from Daiichi Sankyo. J.M. Hill reports personal fees, grants and equity in Shockwave Medical, and personal fees and grants from Abbott Vascular, Boston Scientific and Abiomed. T. Akasaka reports honoraria and grants from Abbott Vascular Japan, and Daiichi-Sankyo Pharmaceutical Inc., and institutional grants from Boston Scientific, Nipro and Terumo. F. Prati reports consultant honoraria from Abbott Vascular and Amgen. H.G. Bezerra reports institutional grant support from Abbott Vascular, and consulting fees and honoraria from Abbott Vascular, Medtronic, and Abiomed. W. Wijns reports institutional grant support from Endotronix and HealthWatch, personal fees from MicroPort, and being a co-founder of Argonauts, an innovation facilitator and medical advisor of Rede Optimus Research. Gary S. Mintz reports honoraria from Boston Scientific, Philips, Terumo, and Medtronic. R.J. McGreevy, Z. Zhang, R.J. Rapoza and N.E.J. West are employees of Abbott Vascular. N.E.J. West is a stockholder in Abbott. G.W. Stone reports speaker or other honoraria from Cook, Terumo, Qool Therapeutics and Orchestra Biomed, being a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, and Matrizyme, and holding equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, and Valfix. O. Ben-Yehuda reports being an employee of the Cardiovascular Research Foundation, which has received research grants from Abbott Vascular. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

