
In the current issue of EuroIntervention, the one-year follow-up of the ILUMIEN III trial1 and the design and rationale of the ILUMIEN IV trial2 are presented.
Both trials are part of a series of clinical studies designed to provide hypothesis-generating knowledge and hypothesis-confirming answers in a systematic way. The prospective, observational ILUMIEN I trial demonstrated that the anatomical vessel information, as provided by optical coherence tomography (OCT), impacts on the clinician’s decision making during percutaneous conronary intervention (PCI) concerning stent size, length and post-dilatation3. The retrospective, post hoc, matched pair ILUMIEN II trial revealed that clinicians can achieve a similar degree of stent expansion when using either OCT or intravascular ultrasound (IVUS) during PCI4. The ILUMIEN III trial confirmed, in a prospective, randomised manner, that clinicians achieve a similar degree of stent expansion when using either OCT or IVUS during PCI. Thus far, there were no surprises with the findings and outcomes: importantly, they provided proof that all observations accumulated over the last three decades for IVUS also hold true for OCT imaging. This seems to speak for itself, considering that both approaches provide cross-sectional, calibrated images of the coronary artery wall. However, this fact warrants explicit mention to assure clinicians, as OCT imaging shows differences to IVUS in image resolution, tissue contrast and imaging procedure with brief blood displacement by X-ray contrast agent5. Invariably, invasive imaging by OCT and IVUS alike provides the clinician with detailed, additional anatomical information, that principally cannot be delivered by angiography. This information is clinically relevant, as it changes the PCI strategy in roughly half of the cases. Indubitably, invasive imaging guidance by OCT and IVUS alike improves stent expansion when compared to angiography (Figure 1). Inevitably, the choice is yours: OCT or IVUS guidance. Both approaches improve acute stent results as compared to angiography, with similar efficiency! When both imaging devices are available in a catheterisation laboratory, the choice should be driven by the experience and training of the operator and cath lab staff, comorbidities of the patient, and lesion characteristics6.
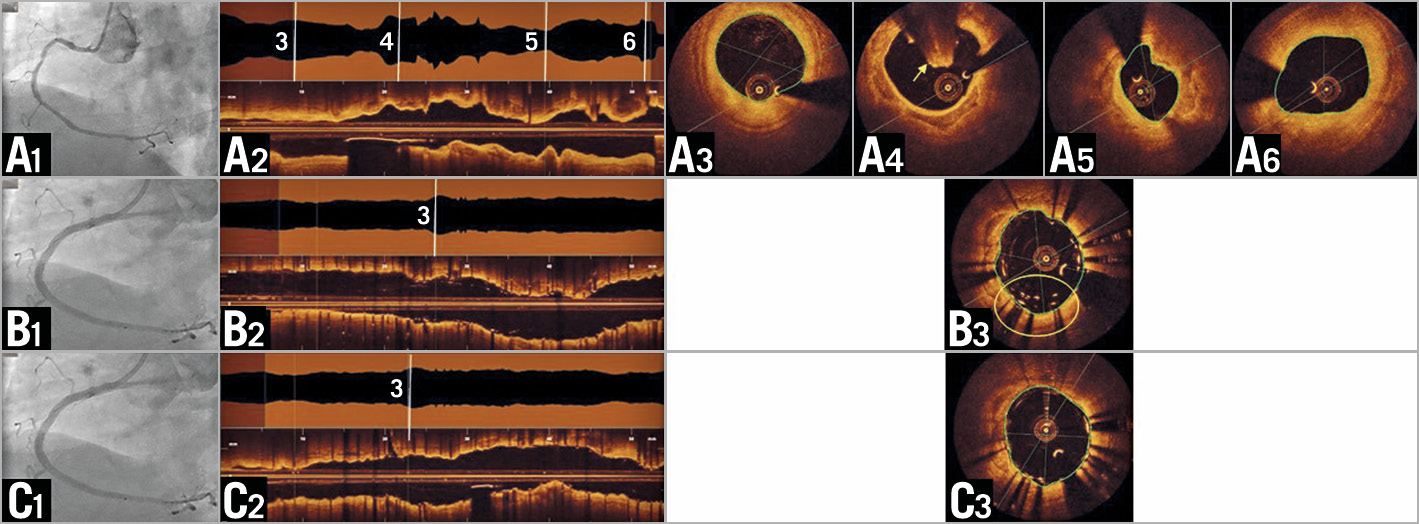
Figure 1. OCT imaging in clinical practice. A) Pre-intervention. A1) Angiography shows diffuse disease without a distinct culprit lesion. OCT (longitudinal reconstruction [A2]) reveals mural thrombus (A4, arrow) and a minimal lumen area of 3.0 mm² (A5). Distal lumen area 6.2 mm² (A3); proximal lumen area 6.3 mm² (A6). B) Post stent implantation. B1) Angiography shows good result. OCT (B2) reveals a region with malapposition of multiple layers of stent struts (B3, circle). C) Final. C1) Angiography appears unchanged. OCT (C2) visualises good strut apposition (C3) and stent expansion.
The acute stent result, namely stent expansion and minimal stent area (MSA), is a key predictor for clinical sequelae and patient prognosis. A substantial number of prospective, randomised clinical trials have shown that IVUS improves prognosis after drug-eluting stent (DES) implantation. Large-scale registries confirmed these findings in daily routine clinical practice, consistently showing a survival benefit in patients with IVUS-guided DES procedure.
ILUMIEN III showed an acute procedural benefit for invasive imaging with OCT or IVUS; however, the trial was not powered to detect prognostic benefit. This is of note when interpreting the one-year follow-up as reported in this issue. The trial designed to test a clinical benefit for OCT-guided DES implantation up to two-year follow-up is the prospective, randomised, multicentre ILUMIEN IV trial. A few points are worthy of note:
– Enriched patient population: key inclusion criteria aim to ensure participation of patients with complex lesions by requiring the presence of medication-treated diabetes mellitus and/or the presence of long or multiple lesions, bifurcation lesions, severe calcification, in-stent restenosis, chronic total occlusion (CTO) (after successful antegrade crossing).
– Adaptive trial design: 2,490 patients with diabetes and/or complex coronary disease will be randomised to OCT versus angiography-guided DES implantation. Sequential blinded interim analyses will be performed for the primary endpoints in order to ensure adequate statistical power: a) for MSA after the first 1,600 patients, to assure the presence of an efficacy signal, namely a larger MSA in the OCT arm than in the angiography arm. If no difference in MSA is present, the trial may be terminated for futility. If superiority in the OCT-guided arm in terms of MSA is demonstrated, then interim analysis will follow b) for target vessel failure (TVF) after 50% of the anticipated events have occurred to determine whether a sample size increase up to 3,656 is needed. The trial will keep enrolling subjects until the target number of TVF events has been adjudicated. Otherwise, the trial may be terminated for futility. At the time of manuscript submission, 1,650 patients had been included in the ILUMIEN IV trial.
– Rigorous training of the operators for OCT image interpretation: intravascular imaging guidance of stenting is a safe, efficient and fast procedure in trained teams. In order to assure an adequate level of expertise, participating centres and operators are being trained intensely and constantly monitored for compliance with the imaging protocol. This is of note, as optimal acute stent results do not depend solely on the imaging technology, but on the conclusions and appropriate actions taken by the operator on the anatomical information it provides.
– ILUMIEN IV OCT criteria: criteria for OCT-guided PCI optimisation in ILUMIEN IV are highly detailed, as is to be expected in a tightly controlled, prospective, randomised, multicentre clinical trial environment. They have two different aims, namely (1) to assess the final stent expansion, and (2) to guide the operator’s actions in order to achieve an optimal stent result.
In order to make such a complex algorithm easily comprehensible, the fundamental principle of optimal stent implantation should be kept in mind, which is in fact very simple: the goal of stenting is to create optimal, laminar flow conditions for the blood within the lumen. This is achieved by eliminating triggers for turbulent flow, such as rapid changes in the geometry or size of the blood flow area, typically caused by inadequate stent expansion, residual narrowing, grossly malapposed stent struts, disease of the reference segments, and edge dissections. In short lesions, such conditions can be summarised by simple criteria, such as MSA >90% of mean reference lumen area. In longer, complex lesions, a single criterion might not be appropriate, as natural vessel tapering or side branch take-offs cause a large difference between proximal and distal reference lumen area.
Therefore, it is not surprising that there are a number of different invasive imaging algorithms in the literature that have proven useful to guide the operators’ procedural strategy. The result that needs to be achieved is always the same: restoration of best flow conditions in any given vessel anatomy.
On a broader perspective, the interventional community is moving towards comprehensive approaches in PCI, integrating anatomical information with physiology and function. It is time to capitalise on the wealth of information produced by invasive imaging over the last three decades. ILUMIEN IV is a prominent example with results eagerly awaited in 2023.
Conflict of interest statement
E. Regar declares being a medical advisor for Zed Medical Inc., USA, and a medical advisor for Kaminari Medical B.V., the Netherlands.
Supplementary data
To read the full content of this article, please download the PDF.

