
Abstract
Aims: In the ILUMIEN III trial, among 450 randomised patients with non-complex lesions undergoing percutaneous coronary intervention (PCI), optical coherence tomography (OCT) guidance led to greater stent expansion than angiography guidance, similar minimal stent area compared to both intravascular ultrasound (IVUS) guidance and angiography guidance, and lower rates of uncorrected dissection and malapposition than both IVUS guidance and angiography guidance. Whether these differences impact on clinical outcomes is unknown. The aim of the present study was to report the 12-month clinical follow-up data from the ILUMIEN III study.
Methods and results: OCT-guided PCI, using an external elastic lamina-based protocol, was compared to operator-directed IVUS-guided or angiography-guided PCI. Target lesion failure (TLF) and major adverse cardiovascular events (MACE) at 12 months were adjudicated by a blinded clinical events committee. There were no significant differences in the rates of TLF (2.0% OCT, 3.7% IVUS, 1.4% angiography), MACE (9.8% OCT, 9.1% IVUS, 7.9% angiography), or any of the individual components of these outcomes among the groups. No independent predictors of 12-month stent-related clinical events were identified from final OCT.
Conclusions: In this underpowered study, OCT-guided PCI of non-complex lesions did not show a statistical difference in clinical outcomes at 12 months compared with IVUS or angiography guidance. An appropriately powered trial, including only complex patients and lesions, is underway to substantiate the potential clinical benefit of OCT-guided PCI. Trial registration: NCT02471586
Introduction
By providing detailed visualisation of the coronary artery lumen and wall, the ability to measure vessel dimensions accurately and to delineate the extent, severity, and type of coronary plaque, intravascular imaging adds valuable information that is complementary to angiography in guiding percutaneous coronary intervention (PCI) procedures1,2. The two most commonly used intracoronary imaging modalities, intravascular ultrasound (IVUS) and optical coherence tomography (OCT), have thus been studied as adjunctive tools to optimise acute procedural results and thereby improve long-term post-PCI outcomes1,2.
Compared with IVUS, OCT is a newer intravascular imaging modality, providing faster acquisition of higher resolution (10-20 μm) images, capable of more accurately identifying thrombus, lipid, calcium, fibrous cap thickness, dissections, plaque prolapse, stent malapposition, and strut coverage2,3,4,5,6. Accumulating data on IVUS-guided PCI have consistently shown superior outcomes compared with angiography guidance in reducing major adverse cardiovascular events (MACE), including target lesion revascularisation, stent thrombosis, and cardiac mortality7,8,9, with continued benefits up to five years8. Although data from registries have shown improved post-PCI clinical outcomes with OCT guidance compared with angiography alone4,5,10, including reduced cardiac mortality4 and lower rates of in-hospital MACE and long-term all-cause mortality10, randomised trial data on the clinical impact of OCT-guided PCI are limited11.
The ILUMIEN III: OPTIMIZE PCI trial was a randomised comparison of PCI with an OCT-guided stent optimisation protocol compared with IVUS-guided and angiography-guided PCI in non-complex lesions. The primary endpoint of the study, the immediate post-procedural minimal stent area (MSA) achieved with OCT guidance, was non-inferior, but not superior, to IVUS guidance and angiography guidance12. OCT-guided PCI led to greater stent expansion than angiography-guided PCI, and lower rates of uncorrected dissection and malapposition than both IVUS-guided and angiography-guided PCI. Whether these improvements in acute procedural parameters translate to improved clinical outcomes is unknown. Herein, we report the 12-month clinical follow-up data from the ILUMIEN III study.
Methods
STUDY DESIGN
The rationale, design, and results of the ILUMIEN III: OPTIMIZE PCI trial have been published previously12. In brief, the ILUMIEN III: OPTIMIZE PCI trial was a prospective, three-arm, single-blind, multicentre trial in which patients undergoing PCI of non-complex lesions with a metallic drug-eluting stent for angina (stable or unstable), silent ischaemia, non-ST-segment elevation myocardial infarction (MI), or recent ST-segment elevation MI (>24 hours from initial presentation) were randomised 1:1:1 to undergo either OCT-guided, IVUS-guided, or angiography-guided stent implantation. A final blinded OCT was performed in the IVUS and angiography arms for comparison of the final MSA between the groups. Eligible patients had one or more target lesions located in a native coronary artery with a visually estimated reference vessel diameter (by angiography) of 2.25-3.50 mm and a length of <40 mm. Patients with left main or ostial right coronary artery stenoses, bypass graft stenoses, chronic total occlusions, planned two-stent bifurcations, and in-stent restenosis were excluded.
A total of 450 patients were enrolled at 29 international centres. Patients randomly allocated to the OCT group were treated according to a specific stent optimisation algorithm based on measurement of the external elastic lamina in the proximal and distal reference segments12. The primary imaging-based outcome (final MSA on OCT) and secondary imaging-based outcomes (including acute procedural success, defined as the percentage of patients achieving optimal [≥95%] or acceptable [90% to <95%] stent expansion; minimum stent expansion; mean stent expansion; tissue or thrombus protrusion; untreated reference segment disease; dissections; and stent malapposition) were assessed by an independent core laboratory (Cardiovascular Research Foundation, New York, NY, USA) blinded to treatment assignment. Target lesion failure (TLF), a composite of cardiac death, target vessel MI, or ischaemia-driven target lesion revascularisation and MACE, a composite of death, MI, stent thrombosis, or repeat revascularisation, were clinical endpoints adjudicated by a clinical events committee blinded to treatment assignment up to 12-month follow-up.
STATISTICAL ANALYSIS
According to the Shapiro-Wilk test, the primary endpoint of ILUMIEN III (MSA) was not normally distributed. Therefore, continuous variables are presented as medians with interquartile ranges and the differences among the groups are examined using the Mann-Whitney U test. Categorical data were compared with the χ2 or Fisher’s exact test. The associations between post-PCI OCT findings and clinical events at one year were assessed using a Cox proportional hazards regression model. Due to the small number of events, multivariable logistic regression was not performed to avoid overfitting the regression model. Event rates were estimated using Kaplan-Meier estimates and were compared using the log-rank test. All statistical tests were two-tailed. Statistical significance was set at a level of 0.05. All analyses were performed with SAS version 9.4 (SAS Institute, Inc., Cary, NC, USA).
Results
CLINICAL, ANGIOGRAPHIC, PROCEDURAL, AND INTRAVASCULAR IMAGING CHARACTERISTICS
Patient assignment and follow-up are shown in Figure 1. A total of 450 patients were randomised in ILUMIEN III: 158 (35%) to OCT guidance, 146 (32%) to IVUS guidance, and 146 (32%) to angiography guidance. At one year, follow-up data were available in 153 (96.8%), 136 (93.2%), and 142 (97.3%) patients, respectively.
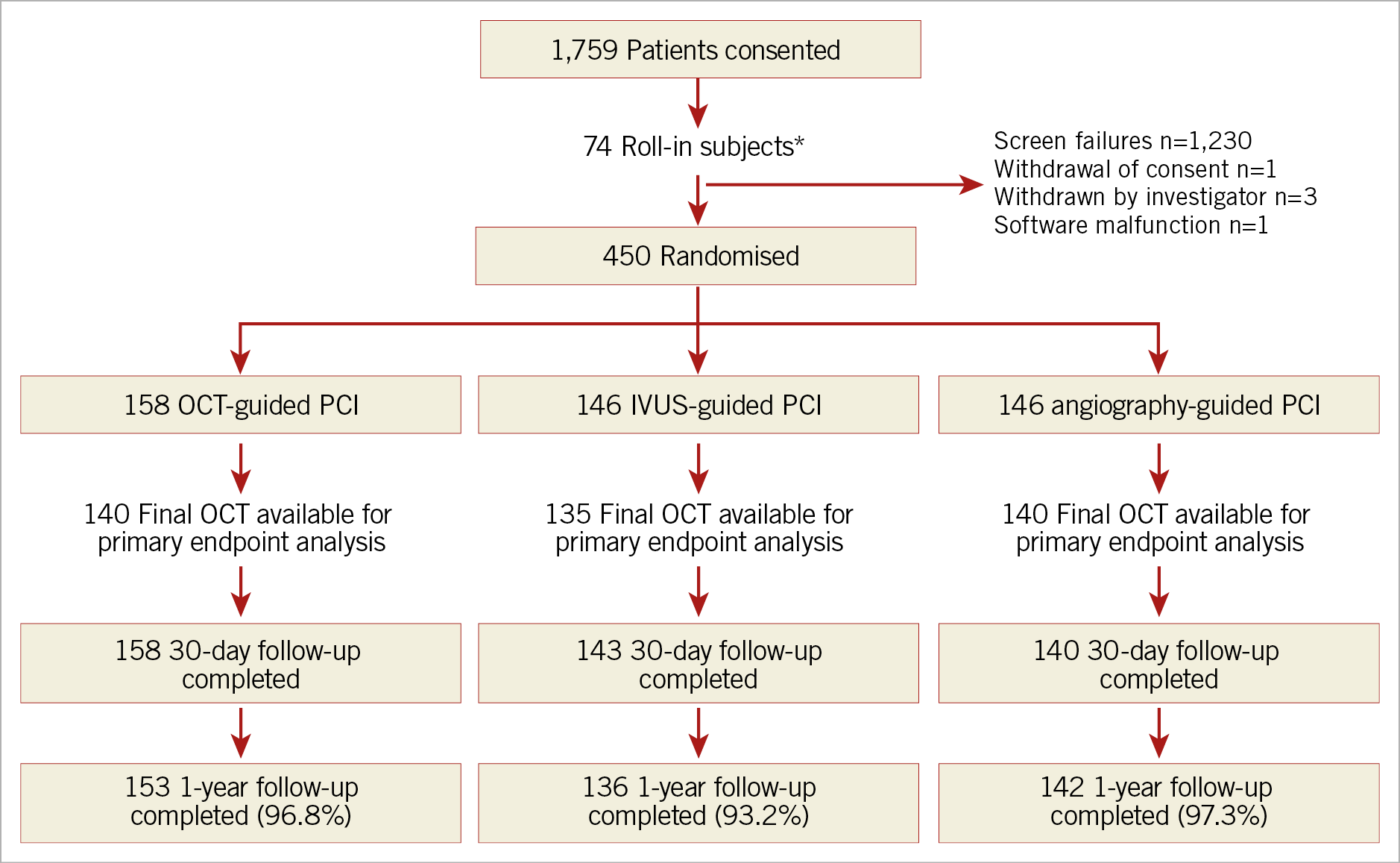
Figure 1. Trial flow diagram. *These non-randomly allocated patients were used to show the investigators’ ability to follow the prescribed OCT guidance procedure. IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention
Baseline clinical characteristics of the patients in whom one-year follow-up was completed were well balanced among the groups (Supplementary Table 1). Angiographic and procedural characteristics are summarised in Supplementary Table 2. There were no differences in the pre-PCI angiographic distribution, complexity, or severity of coronary lesions among the groups. Moreover, the number of stents and stent length and diameter were similar among the groups.
Intravascular imaging guidance by either OCT or IVUS resulted in more frequent post-dilatation, use of larger maximum balloon sizes, and higher balloon inflation pressure compared with angiography guidance. Procedure duration was longer in the two intravascular imaging arms, although there were no significant differences in fluoroscopy times or radiation dose. Contrast media use was greatest in the OCT-guided arm.
As measured by final OCT, the final median post-PCI MSA was 5.79 mm2 (4.58-7.34) with OCT guidance, 6.20 mm2 (4.69-7.92) with IVUS guidance, and 5.49 mm² (4.39-6.59) with angiography guidance. The MSA with OCT guidance was non-inferior to IVUS guidance (Supplementary Table 3). Nevertheless, minimal and mean stent expansion were significantly greater with OCT-guided PCI than with angiography-guided PCI and were similar to IVUS-guided PCI. The proportion of patients with procedural success was higher with OCT-guided and IVUS-guided PCI than with angiography-guided PCI (Supplementary Table 3). On the final blinded OCT, untreated edge dissections were more frequently present after IVUS-guided and angiography-guided PCI than after OCT-guided PCI (Supplementary Table 3). Untreated major dissections were more common after IVUS-guided PCI than after OCT-guided PCI. Similarly, untreated major stent malapposition after PCI was more frequent with both IVUS and angiography guidance than with OCT guidance.
CLINICAL OUTCOMES AT 12 MONTHS
At 12 months, the rates of TLF were not different among the three groups: 2.0% with OCT guidance, 3.7% with IVUS guidance, and 1.4% with angiography guidance (Table 1, Figure 2A). The individual components of TLF were also not different among the groups. At 12 months, the rates of MACE were not different among the three groups: 9.8% with OCT, 9.1% with IVUS, and 7.9% with angiographic guidance (Table 1, Figure 2B). The individual components of MACE were also not different among the groups.
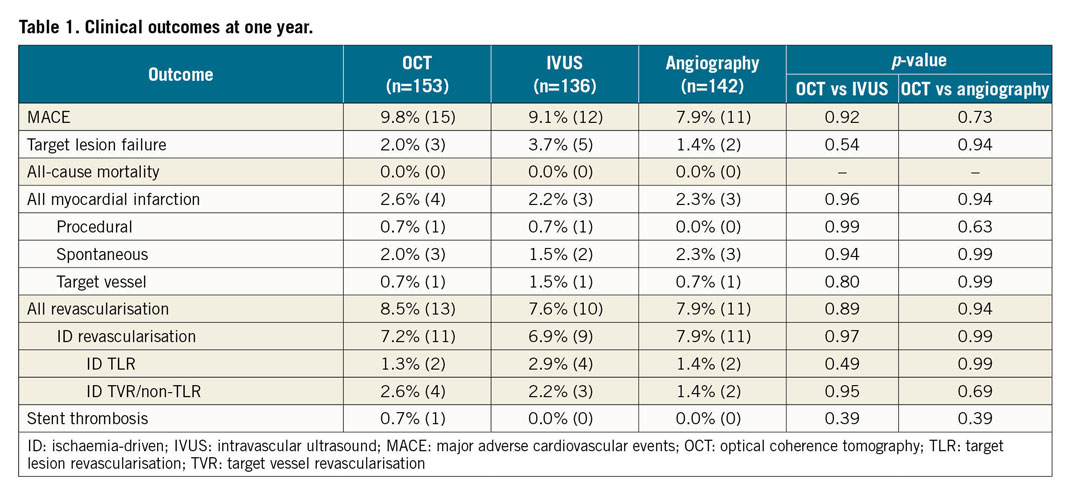
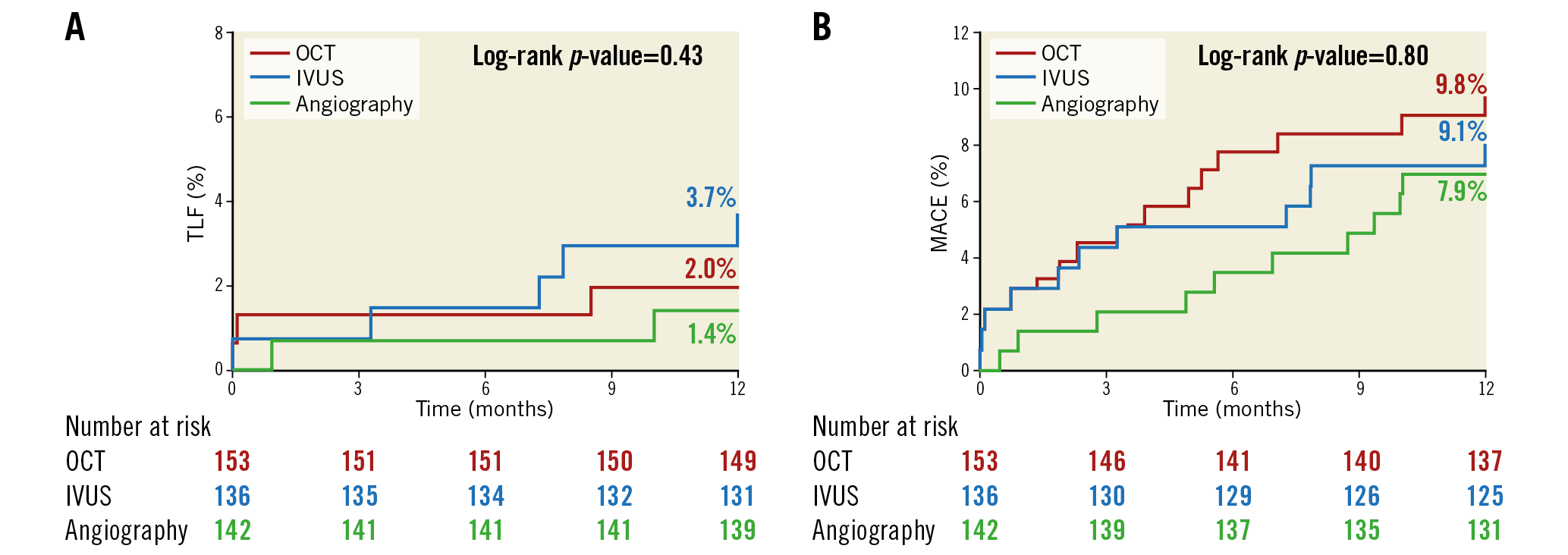
Figure 2. Time-to-first-event curves for target lesion failure and major adverse cardiovascular events up to one-year follow-up. A) The rates of target lesion failure (composite of cardiac death, target vessel myocardial infarction, or ischaemia-driven target lesion revascularisation). B) The rates of major adverse cardiovascular events (composite of death, myocardial infarction, stent thrombosis, or repeat revascularisation). Event rates were based on Kaplan-Meier estimates. IVUS: intravascular ultrasound; OCT: optical coherence tomography
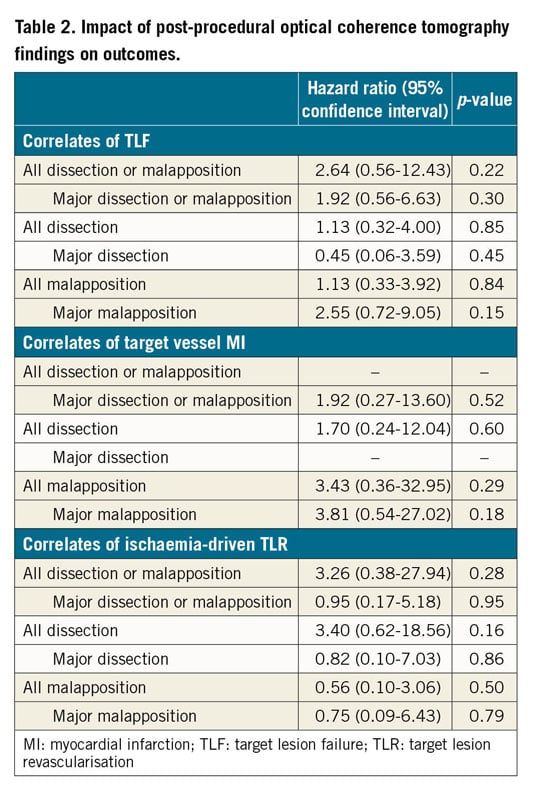
The associations between post-procedure OCT findings and clinical outcomes are summarised in Table 2. No OCT findings were statistically significant predictors of stent-related clinical events at 12 months.
Discussion
ILUMIEN III: OPTIMIZE PCI was the first randomised controlled trial to use a specific OCT-based protocol to determine stent length and diameter according to external elastic lamina measurements in order to optimise lumen dimensions and lesion coverage, which compared post-PCI MSA with IVUS and angiographic guidance. Post-PCI MSA achieved after OCT-guided PCI was non-inferior to that achieved with IVUS-guided PCI, meeting the primary endpoint of the trial. OCT-guided PCI resulted in significantly greater minimal and mean stent expansion than did angiography-guided PCI, with greater procedural success12. There were no significant differences between OCT-guided PCI and IVUS-guided PCI in terms of MSA. Nevertheless, OCT guidance resulted in fewer untreated major dissections than did IVUS guidance and fewer areas of major stent malapposition than did both IVUS guidance and angiography guidance. This follow-up study of the ILUMIEN III trial reports no significant differences in the rates of TLF or MACE among the three arms at 12 months.
There may be several possible explanations for the findings of the current study. Most importantly, ILUMIEN III was not statistically powered to detect the impact of the PCI guidance strategies on the rates of TLF or MACE. Therefore, the inferences drawn from this report should be considered hypothesis-generating. In addition, the lesions enrolled in ILUMIEN III were intentionally non-complex to demonstrate the feasibility of the use of external elastic lamina measurements to guide OCT stent optimisation. The event rates, and thus the power to be able to demonstrate differences among the three imaging guidance strategies, would be greater with enrolment of more complex lesions and patients. Indeed, in larger IVUS studies the clinical benefits of imaging-guided PCI appear robust and durable7,8,9.
In the OPINION study11, the only prior randomised trial (n=817) to compare the impact of IVUS guidance versus OCT guidance on post-PCI clinical outcomes, there was no difference in the combined endpoint of cardiac death, target vessel MI, or target vessel revascularisation at 12 months (5.2% in the OCT-guided group and 4.9% in the IVUS-guided group, p=0.0042 for non-inferiority). Nevertheless, the event rates in the OPINION trial were lower than the 9% assumed in the power calculation, probably due to the exclusion of patients with high-risk clinical features (e.g., MI within the past three months) and complex lesions (e.g., chronic total occlusion, in-stent restenosis, small target vessels), indicating the critical importance of accurately estimating event rates based on the included patient population and target lesion characteristics in the OCT-based PCI strategy trials to ensure adequate statistical power.
Conversely, in an analysis of a large prospective observational cohort consisting of an “all-comers” population of patients undergoing PCI (excluding those presenting with ST-segment elevation MI or those undergoing pressure wire-guided PCI) that included 87,166 patients (1,149 with OCT guidance, 10,971 with IVUS guidance, and 75,046 with angiography guidance), OCT-guided PCI was associated with reduced rates of in-hospital MACE (0.8% versus 2.0%, p=0.01) and all-cause mortality compared with angiography-guided PCI (9.6% versus 16.8%, p<0.0001) but not significantly with IVUS-guided PCI (8.9% versus 10.2%, p=0.12) during the follow-up period10. Taken together, these two studies emphasise that randomised trials aiming to detect the clinical benefits of OCT guidance on clinical outcomes compared with angiography alone need to ensure adequate statistical power by enrolling a large number of patients enriched with complex lesions and other high-risk clinical features.
The ILUMIEN III trial met its primary endpoint of non-inferiority of OCT guidance compared to IVUS guidance in post-PCI MSA (with a 1.0 mm2 non-inferiority margin). Post-PCI MSA is the most important determinant of freedom from early and late MACE after stenting4,13,14. Thus, the fact that there were similar rates of MACE among the three arms at 12 months is consistent with all three strategies achieving similar post-procedural MSAs.
Conversely, in the present study, differences observed in the post-PCI findings among the three arms, i.e., fewer areas of major stent malapposition with OCT guidance compared with both IVUS guidance and angiography guidance and fewer untreated major dissections with OCT guidance than IVUS guidance, did not impact on stent-oriented outcomes or MACE. While this may reflect the lack of power in the present study, there is no established link between acute post-PCI malapposition and subsequent stent failure rates, unless the malapposition is also associated with stent underexpansion15. Furthermore, although large IVUS-detected stent edge dissections have been strongly associated with subsequent stent thrombosis and restenosis16, the severity of OCT-detected stent edge dissections that might have similar or greater prognostic utility has not been firmly established17. While an observational study suggested an association between stent edge dissections >310 μm in width with subsequent stent restenosis and stent thrombosis in acute coronary syndromes18, other studies have not found such an association6,17,19,20. In contrast, the length, width, and arc of distal edge dissection have been shown to be associated with MACE in registries, with either distal edge dissection >200 μm in width4,5 or length of >3.55 mm21 being independent predictors of device-oriented adverse events at one year4,21 or two years5. Nonetheless, most non-flow-limiting OCT-detected edge dissections are resolved on serial OCT imaging at 6 to 12 months without causing stent failure17,20,21,22,23.
Most complex lesions, including very long lesions, in-stent restenosis, bifurcation lesions, and chronic total occlusions, were excluded from the ILUMIEN III trial. The superior resolution of OCT may have a greater clinical impact in this complex subset of lesions in which the risk of stent failure is higher than the non-complex lesions included in the ILUMIEN III trial, especially in high-risk patient populations such as diabetics and in those with acute coronary syndromes24.
There were no intravascular imaging-related complications in the OCT or IVUS arms in the present study. Previous studies have also shown complications related to intravascular imaging to be rare (≈0.5%) and readily manageable in the catheterisation laboratory25. Nonetheless, careful technique when performing intravascular imaging is paramount to minimise the risk of complications related to instrumentation of coronary arteries with the imaging catheters, an inevitable hazard that is not applicable to angiography-guided PCI.
Limitations
ILUMIEN III was not statistically powered to detect the impact of the strategies for PCI guidance on the rates of TLF or MACE, and by design excluded the complex coronary artery lesions. Thus, the findings of the present one-year follow-up report are to be considered hypothesis-generating.
Conclusions
The findings of the ILUMIEN III trial and previous OCT-based studies have laid the foundation for the ongoing ILUMIEN IV trial (NCT03507777), a prospective, single-blind study randomising between 2,490 and 3,656 patients with complex lesions or high-risk clinical features to OCT-guided versus angiography-guided coronary stent implantation in a 1:1 ratio. The primary endpoints are (1) post-PCI MSA assessed by OCT in each randomised arm, and (2) target vessel failure, defined as the composite time-to-first-event rate of cardiac death, target vessel MI, or ischaemia-driven target vessel revascularisation. Thus, ILUMIEN IV is the first adequately powered trial to test rigorously the hypothesis that better morphologic lesion characterisation with enhanced procedural planning and stent optimisation effected by OCT guidance compared with angiography guidance will result in improved acute procedural results and thus early and late clinical outcomes.
|
Impact on daily practice This study reports the 12-month clinical follow-up data from the ILUMIEN III trial which investigated OCT-guided PCI using a specific protocol based on reference segment external elastic lamina measurements compared with operator-directed IVUS-guided or angiography-guided PCI. Although underpowered to detect differences, OCT-guided PCI did not impact on clinical outcomes at 12 months compared with IVUS or angiography guidance. The adequately powered ILUMIEN IV trial is ongoing to examine whether OCT-guided versus angiography-guided PCI provides substantial long-term clinical benefits. |
Funding
The ILUMIEN III trial was funded by St. Jude Medical (St. Paul, MN, USA).
Conflict of interest statement
Z.A. Ali reports institutional research grants to Columbia University and the Cardiovascular Research Foundation from Abbott and Cardiovascular Systems Inc., being a consultant for Abbott, Abiomed, Acist Medical, Amgen, AstraZeneca, Boston Scientific, Cardinal Health, and Opsens Medical, and holding equity in Shockwave Medical. A. Maehara reports institutional grant support from Abbott Vascular and Boston Scientific, and reports being a consultant for Conavi Medical Inc. G. Guagliumi reports institutional research grant support from Abbott Vascular, Boston Scientific, and Infraredx, and being a consultant for Abbott Vascular and Boston Scientific. T. Akasaka reports honoraria and grants from Abbott Vascular Japan, and institutional grants from Boston Scientific, Nipro, and Terumo. M. Matsumura reports being a consultant for Terumo Corporation. G.S. Mintz reports honoraria from Boston Scientific, Philips, Terumo, and Medtronic. Z. Zhang: reports being an employee of Abbott. R.J. Rapoza reports being an employee of Abbott. N.E.J. West reports being an employee/stockholder of Abbott. G.W. Stone reports receiving speaker or other honoraria from Cook, Terumo, Qool Therapeutics and Orchestra Biomed, being a consultant to Valfix, TherOx, Vascular Dynamics, Robocath, HeartFlow, Gore, Ablative Solutions, Miracor, Neovasc, V-Wave, Abiomed, Ancora, MAIA Pharmaceuticals, Vectorious, Reva, and Matrizyme, and holding equity/options from Ancora, Qool Therapeutics, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWave, Orchestra Biomed, Aria, Cardiac Success, MedFocus family of funds, and Valfix. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

