
Abstract
Aims: The feasibility of offline optical coherence tomography (OCT) guidance in bifurcation (with either two-dimensional or three-dimensional images) and its potential benefits have been demonstrated in retrospective studies; however, these have not yet been investigated prospectively. The aim of this trial is to determine the superiority of online three-dimensional optical frequency domain imaging (3D-OFDI)-guided stenting to angiography-guided percutaneous coronary intervention (PCI) in terms of incomplete stent apposition (ISA) at the bifurcation segment.
Methods and results: The OPTIMUM trial is a randomised, superiority, multicentre clinical trial. The primary endpoint of this trial is the post-procedural percentage of malapposed struts assessed by OFDI in the main branch bifurcation region after final kissing balloon dilatation (FKBD). A total of 106 patients will be randomly allocated to either 3D-OFDI guidance or angiography guidance PCI. Bifurcation lesions will be treated with a provisional single-stent strategy using the Ultimaster sirolimus-eluting stent. Patients randomised to the 3D-OFDI guidance arm will undergo OFDI assessment in the main branch (MV) after rewiring into the jailed side branch following stent implantation, while in the angiography guidance arm re-crossing of a wire into the side branch will be performed using conventional fluoroscopic/angiographic guidance. In patients in the 3D-OFDI guidance arm, if the position of the wire is not located in the optimal cell, further attempts to redirect the wire to the optimal cell will be performed, with subsequent OFDI acquisitions to confirm the re-crossing position. The proximal optimisation technique and FKBD are mandatory in this trial. The study will provide a 90% power to show superiority of 3D-OFDI guidance PCI compared with angiography-guided PCI.
Conclusions: The OPTIMUM trial will be the first prospective, randomised trial to evaluate the efficacy and safety of online 3D-OFDI-guided PCI in bifurcation lesions. ClinicalTrials.gov Identifier: NCT02972489
Introduction
In bifurcation percutaneous coronary intervention (PCI), re-crossing the distal cell with a wire after main vessel stenting is important to avoid creating a de novo metal carina1. Those protruded/malapposed struts result in lower strut coverage of the side branch ostium and more overhanging metal into the main branch over time after implantation of the stent2. In a phantom model, it has been clearly shown that, after proximal cell rewiring with a kissing balloon, malapposed struts were observed in front of the side branch, while, after distal cell rewiring with a kissing balloon, the struts were well apposed to the vessel wall. Computational flow simulation of the shear rate showed that proximal re-crossing of malapposed struts in front of a side branch could lead to an abnormally high shear rate level above 2,000 cm/s, whereas after distal re-crossing with a kissing balloon less shear rate disturbance was observed2 (Figure 1). Foin et al reported that incomplete stent apposition (ISA) not only affects blood flow patterns as assessed by shear rate calculated in computational fluid dynamic (CFD) simulation but also delays the process of strut coverage3. It is known that ISA is frequently observed using an invasive intracoronary imaging device in patients with late thrombosis3. Hence, it is deemed important to avoid ISA in bifurcations. Angiography-guided PCI is limited in recognising the re-crossing position, while intracoronary image-guided PCI has the potential to guide PCI by confirming the re-crossing point (Figure 2). Moreover, three-dimensional (3D) optical coherence tomography (OCT) images are helpful to understand the geometry of complex coronary lesions and the complex interaction between the coronary devices.
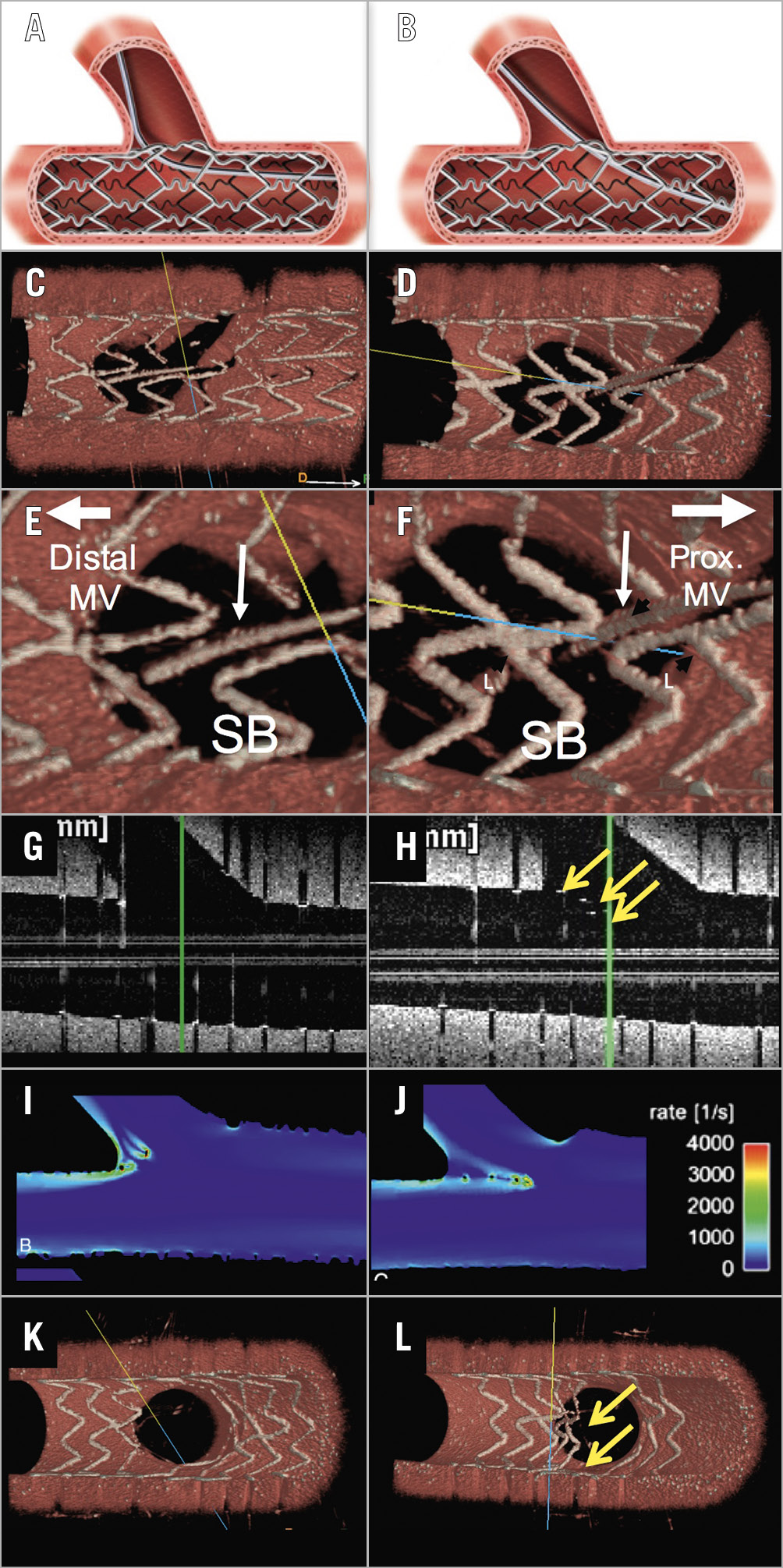
Figure 1. The difference in stent configuration between distal and proximal cell rewiring with kissing balloon inflation. In a phantom model, a stent was implanted in a main vessel. In panel A the wire was re-crossed through a distal cell, while in panel B the wire was re-crossed through a proximal cell. This was confirmed by 3D-OCT (C, D, E, & F). After kissing balloon inflation, in the distal cell rewiring case, the ostium of the side branch was widely open without malapposed struts (G & K), while, in the proximal cell rewiring case, the malapposed struts (yellow arrows) were observed in front of the side branch as assessed by OCT (H & L). The computational flow simulation showed a high shear rate by the malapposed struts in front of the side branch (I, J). 3D-OCT confirmed the malapposed struts in front of the side branch after proximal cell rewiring with kissing balloon inflation, while, after distal cell rewiring with kissing balloon, the ostium of the side branch was widely open. Reproduced with permission from Onuma et al2.
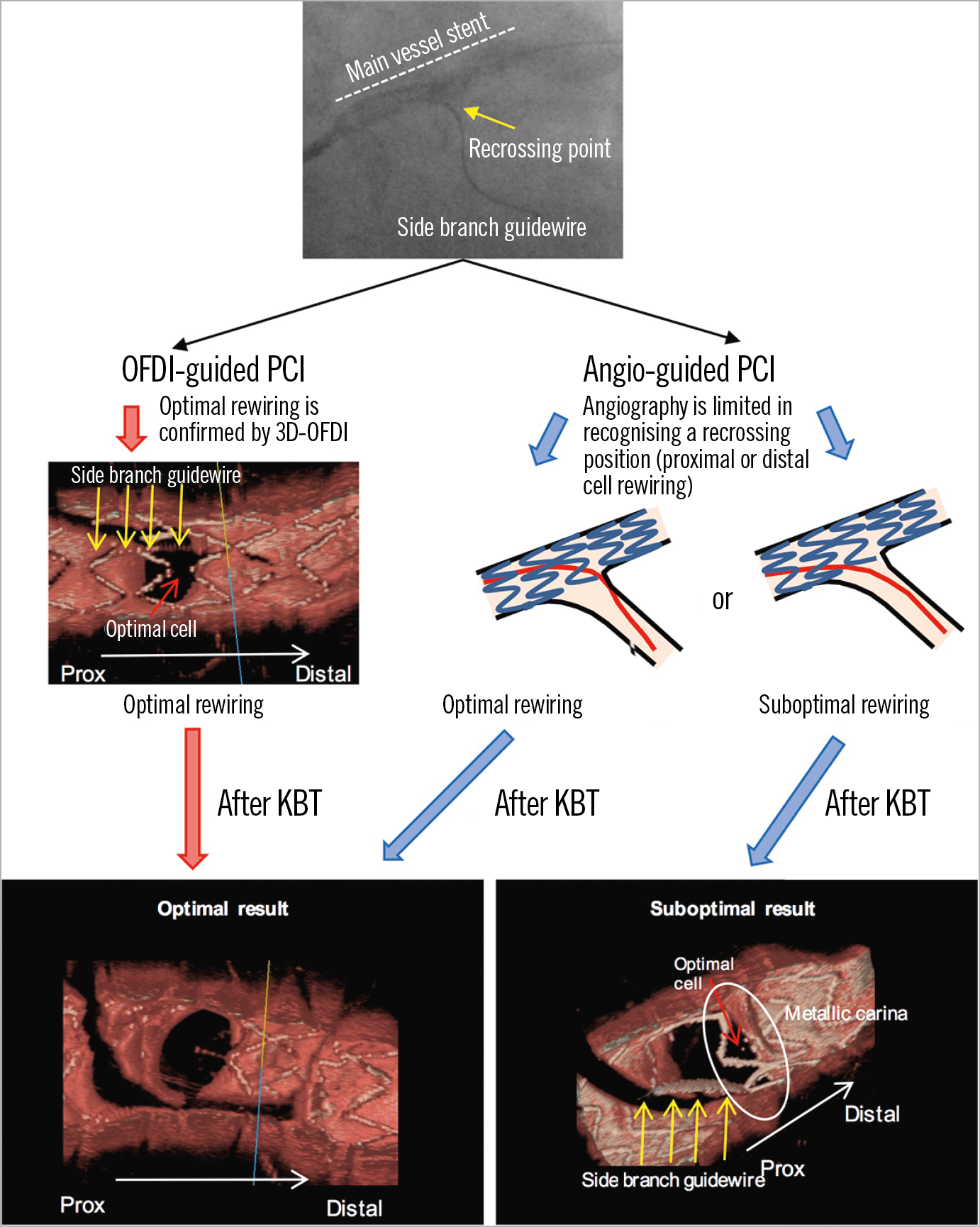
Figure 2. Angio-guided PCI is limited in recognising a re-crossing position. Angio-guided PCI is limited in recognising a re-crossing position whether it is a proximal or distal cell, while OFDI-guided PCI can confirm the re-crossing position. Hence, angio-guided PCI would lead to either an optimal or a suboptimal result, while OFDI-guided PCI would lead to an optimal result.
The recently developed optical frequency domain imaging (OFDI) technique provides higher image acquisition speeds, greater penetration depth, and a higher quality of image resolution. However, there is no randomised clinical trial comparing 3D-OFDI-guided PCI with angiography-guided PCI in bifurcation lesions to prove the superiority of OFDI-guided PCI in bifurcation lesions in terms of the reduction of ISA. Therefore, the aim of the OPTIMUM multicentre, open-label, prospective randomised investigator-driven trial is to determine whether bifurcation stenting guided by online 3D-OFDI is superior to that with angiographic guidance in terms of acute ISA in the bifurcation segment.
Methods
TRIAL DESIGN AND STUDY ENDPOINTS
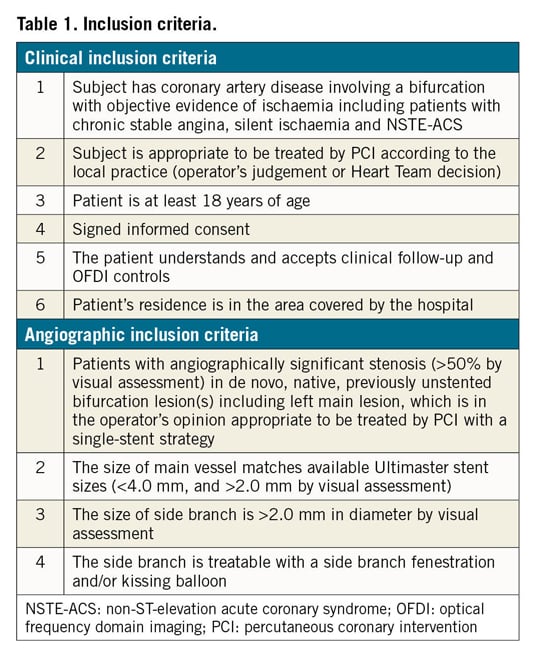
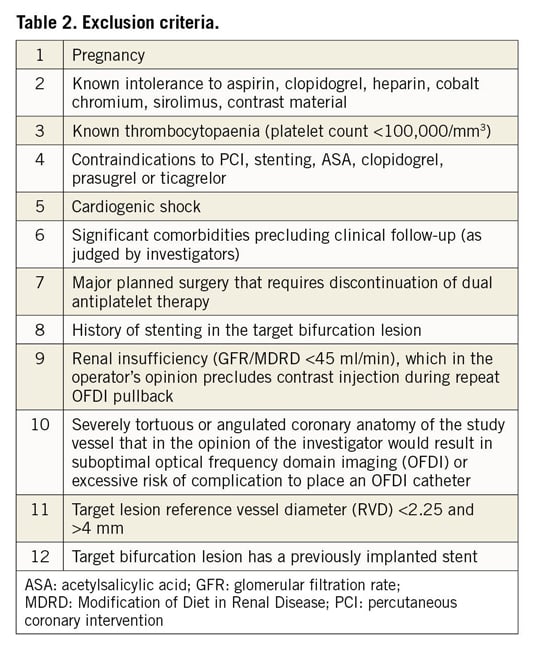
The OPTIMUM (online three-dimensional OPTical frequency domain IMaging to optimise bifurcation stenting using UltiMaster stent) trial is a randomised (1:1; 3D-OFDI-guided vs angiography-guided), active control, superiority, multicentre clinical trial. One hundred and six patients will be enrolled at four Japanese centres (Fujita Health University Hospital, Yamaguchi University Hospital, Osaka Police Hospital and Teikyo University Hospital). Inclusion and exclusion criteria are shown in Table 1 and Table 2, respectively.
The primary endpoint of the trial is the post-procedural percentage of malapposed struts assessed by OFDI in the main branch bifurcation region, which will be calculated as the ratio of the number of malapposed struts to the number of struts in the bifurcation region4 (Figure 3). Malapposed struts include non-apposed struts at the side branch (SB) orifice. Struts located at the ostium of side branches, with no vessel wall behind, are defined as non-apposed struts at the SB orifice4.
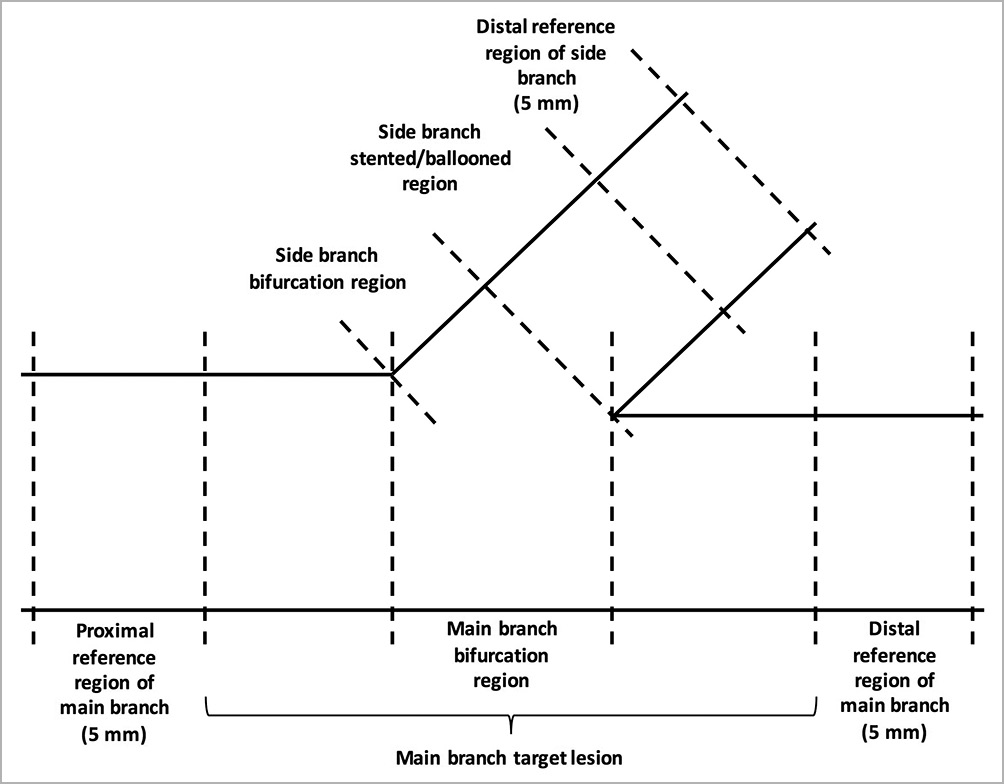
Figure 3. OFDI core lab analysis. The post-procedural percentage of malapposed struts (primary endpoint) will be assessed in the main branch bifurcation region.
Secondary OFDI endpoints at post procedure are as follows: in the entire main branch, 1) percentage of malapposed struts, 2) incomplete stent apposition area, 3) minimum/mean lumen area, 4) minimum/mean stent area, 5) mean/maximum protrusion area, 6) mean/maximum intra-stent defect attached to/free from the vessel wall, and 7) minimum/mean flow area; in the bifurcation region, 1) incidence of fulfilling optimal re-crossing criteria on 3D-OFDI, 2) ISA area, 3) minimum/mean lumen area, 4) minimum/mean stent area, 5) mean/maximum protrusion area, 6) mean/maximum intra-stent defect attached to/free from the vessel wall, and 7) minimum/mean flow area. An intra-stent defect attached to the wall is defined as an irregularly shaped tissue attached to the luminal surface and an intra-stent defect free from the wall is defined as an isolated structure in the lumen distant from the vessel wall5.
Clinical safety endpoints of the trial are as follows: 1) target vessel failure (TVF), defined as cardiac death, reinfarction in the territory of the infarct-related vessel (Q-wave and non-Q-wave), or clinically driven target vessel revascularisation – and its individual components – at discharge, six months and 12 months, 2) all-cause mortality, 3) cardiac death, non-cardiovascular death, vascular death, 4) any myocardial infarction, 5) target lesion and vessel revascularisation, 6) stent thrombosis according to the Academic Research Consortium definition at discharge, at six months and 12 months, and 7) other serious adverse events at discharge, six months and 12 months.
Procedures
PATIENT INFORMATION AND INFORMED CONSENT
Prior to the study start, the investigator must obtain written institutional review board/ethics committee approval for the informed consent form. A copy of the patient information and the signed and dated informed consent will be provided to the study patient. Study patients will be assured that they may withdraw from the study at any time and for any reason.
RANDOMISATION
Before randomisation, the operator verifies the vessel requirement for inclusion after administration of nitrate. If the lesion(s) does not fulfil the inclusion criteria, the subject cannot be randomised. Randomisation is performed via web-based software with random blocks according to centre. Randomisation occurs at the time of the index procedure after successful wiring of the main branch of the bifurcation lesion.
INDEX PROCEDURE
All bifurcation stenting is in principle to be treated with a provisional single-stent strategy. It is strongly recommended to treat all lesions in the target vessel in the same procedure. Staged procedures are allowed but should be completed within six weeks. Moreover, all the staged procedures should be declared at the time of or after the index procedure. All patients will receive the Ultimaster® sirolimus-eluting stent(s) (Terumo Corp., Tokyo, Japan) in the main vessel (MV). After stenting of the MV, the proximal optimisation technique (POT) is mandatory with a 0.25-0.5 mm larger balloon than the device size following the recommendation from the European Bifurcation Club6. Patients randomised to the 3D-guidance arm will undergo OFDI assessment in the MV after rewiring into the jailed side branch following stent implantation. If the position of the wire is not located in the optimal cell, further attempts to redirect the wire to the optimal cell will be performed, with subsequent OFDI acquisitions to confirm the position in 2D and online 3D reconstruction (Moving image 1). The final re-crossing position will be recorded based on the online 3D-OFDI image. In the angiography-guided group, wire re-crossing into the side branch will be performed using conventional fluoroscopic/angiographic guidance. Stent size will be determined by the distal reference diameter of the MV. Both groups will receive final kissing balloon dilatation (FKBD) using balloons matching the size of the SB and of the distal MV. After performing FKBD, OFDI will be acquired to evaluate the primary endpoint for both the OFDI- and angiography-guided PCI groups (Figure 4). Although performing the additional procedure after FKBD will be allowed for safety reasons, the primary endpoints must be evaluated by the OFDI acquired before the additional procedure (i.e., immediately after FKBD). Investigators performing the index procedure are not blinded, as the treatment strategy is clearly identifiable.
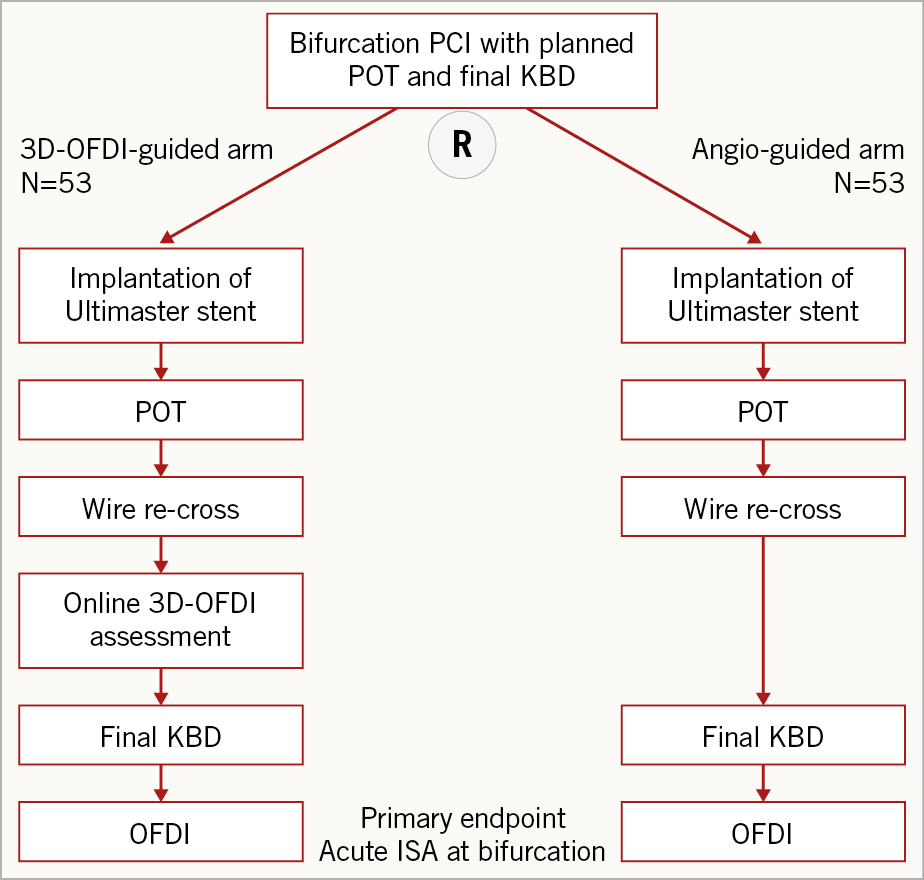
Figure 4. Study flow chart.
In case a second stent at side branches is needed due to dissection, slow-flow, residual stenosis (>75%), etc., the additional stenting can be performed at the operators’ discretion. The procedure will be followed for T-stenting or the TAP technique. Details of the PCI procedures including balloon diameters, stent type and size should be recorded. Stent optimisation and FKBD will be performed with non-compliant balloons.
RE-CROSSING THE OPTIMAL CELL
The optimal re-crossing point is defined according to the configuration of overhanging struts in front of the side branch ostium. When the most distal stent ring is in contact with the rim of the carina, a second distal compartment is considered as the optimal cell. In the presence of multiple second distal compartments, a larger compartment should be selected (Figure 5). Figure 6 shows a suboptimal case, in which the guidewire was re-crossed through the proximal cell. 3D-OFDI clearly detected the protruded/malapposed metallic carina and the overlapping struts created after kissing balloon inflation. In case suboptimal cell re-crossing is observed, redirection of the guidewire is required, in which 3D-OFDI can confirm whether the re-crossing point is optimal (Figure 7, Moving image 1). This is the advantage of the 3D-OFDI-guided PCI in bifurcation in terms of avoiding suboptimal re-crossing. In addition, we have to take into account the importance of the configuration of overhanging struts in front of the ostium, as reported by Okamura et al7. The presence of a longitudinal link in front of the ostium led to more malapposed struts compared to cases without a longitudinal link (Figure 8). In those cases classified as configuration 3 in the current study, in order to reduce the protruded/malapposed struts, a larger compartment was defined as an optimal cell (Figure 5).
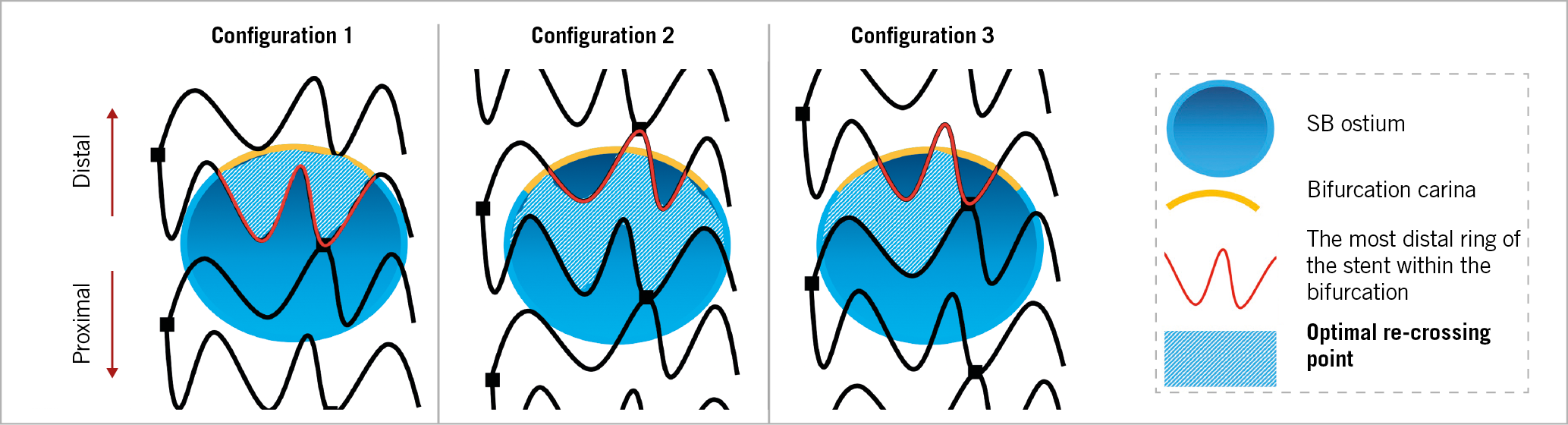
Figure 5. Optimal re-crossing point. Configuration 1: when the most distal stent ring is in contact with the rim of the carina, a second distal compartment is considered as the optimal cell. Configuration 2 & 3: in the presence of multiple second distal compartments, a larger compartment should be selected.
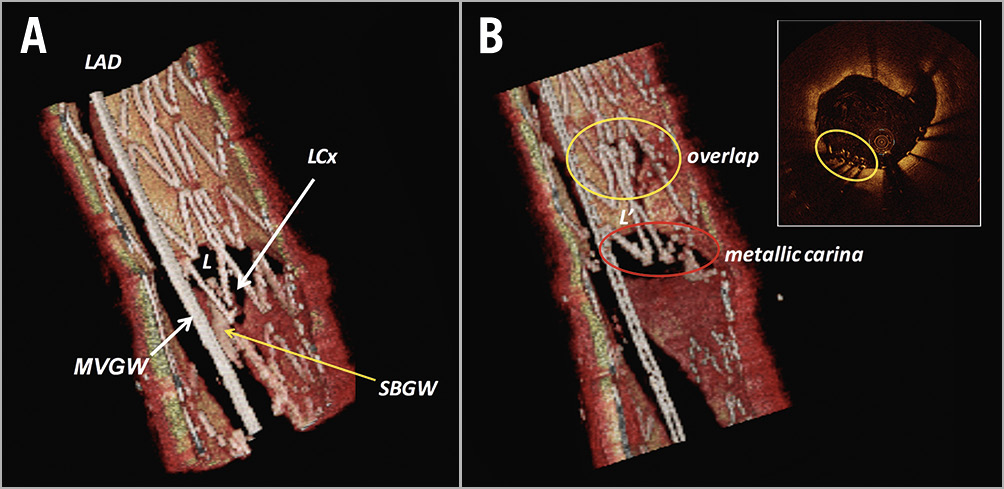
Figure 6. Metallic carina created after proximal cell rewiring with kissing balloon inflation. A) 3D-OCT images show that the re-crossing wire was located in the proximal cell (suboptimal case). The jailed stent struts are pushed to the distal side of the vessel due to the dilatation of the balloon at the orifice of the side branch, causing overlapping of struts (yellow circle) and also a metallic neocarina (red circle) (B). Reproduced with permission from the OCT Atlas (second edition).
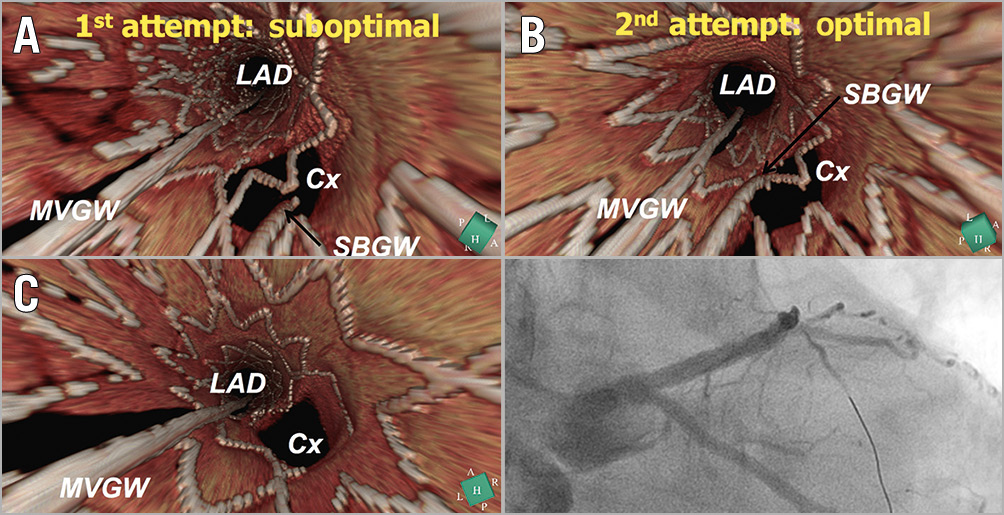
Figure 7. A case example of 3D-OCT-guided PCI. A) The 3D-OCT image indicates that the guidewire re-crossed through the proximal cell at the first attempt at re-crossing the wire. B) The 3D-OCT image shows that the guidewire re-crossed the optimal cell (i.e., the most distal cell) at the second attempt at re-crossing the wire. C) The ostium of the left circumflex was well opened and no ISA was observed. Reproduced with permission from Okamura et al7.
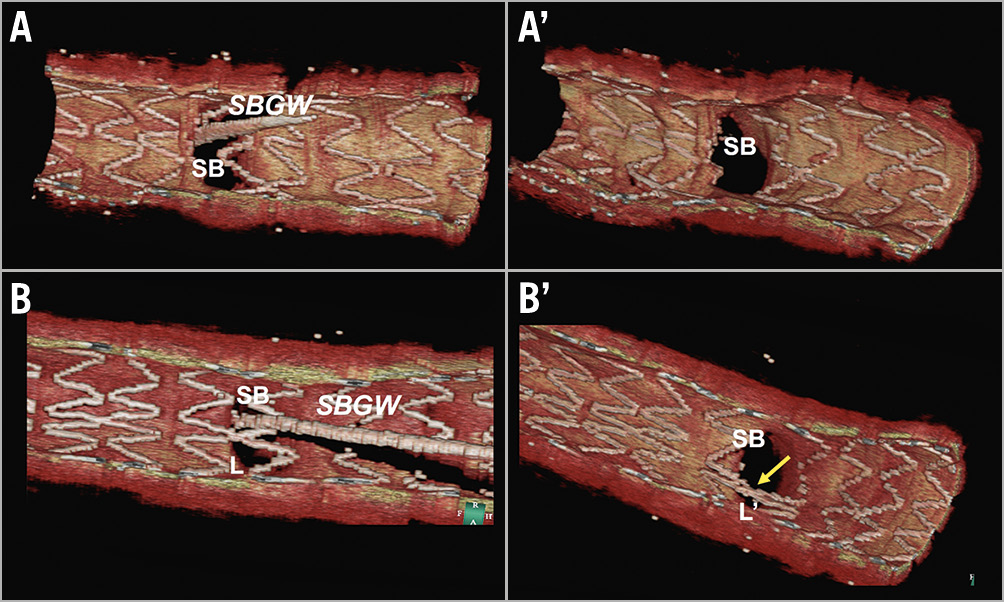
Figure 8. Incomplete stent apposition and the configuration of overhanging struts in front of the ostium. In configuration 1 (without a link in front of the ostium) with distal rewiring (A), no ISA was observed in front of the side branch ostium (A’). In configuration 3, even though distal rewiring was achieved (B), malapposed struts were detected (B’). Reproduced with permission from the OCT Atlas (second edition).
ONLINE THREE-DIMENSIONAL RECONSTRUCTION
The Terumo OFDI console (Figure 9) incorporates online software of 3D imaging with a possibility of stent enhancement, which enables the online reconstruction within one minute (Moving image 2). This console can provide not only a vessel view but also a carpet view. These views give operators detailed information, which could help to understand complex coronary lesions and the complex interaction between the coronary devices and the vessels.
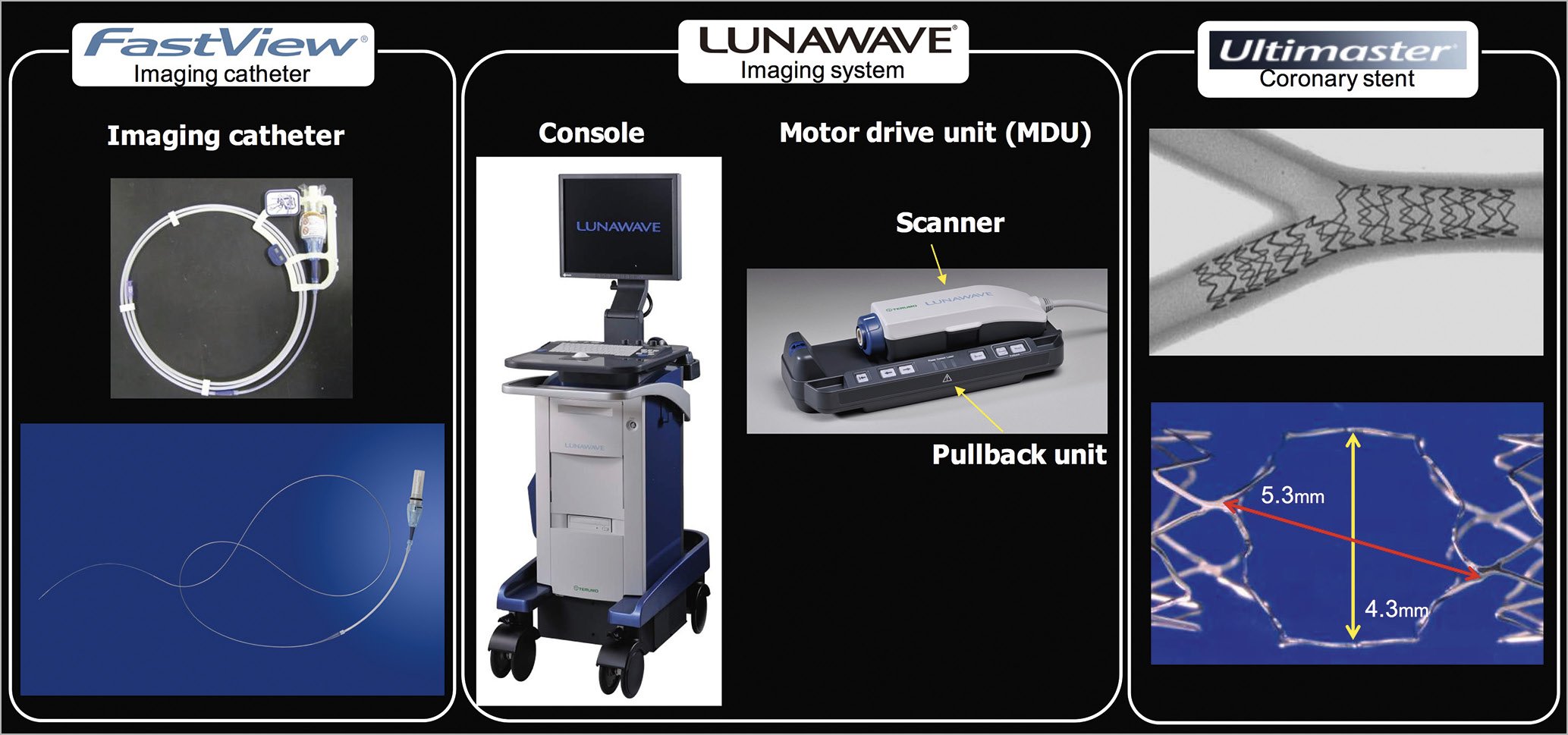
Figure 9. Terumo OFDI system and Ultimaster coronary stent. Left panel: pictures of imaging catheter. Middle panel: pictures of imaging system. Right panel: Ultimaster stent after kissing balloon inflation in a bifurcation model (upper) and potential cell opening in 3.5 mm device (lower). Reproduced and adapted with permission from the OCT Atlas (second edition).
ULTIMASTER STENT
The Ultimaster coronary stent system consists of a cobalt-chromium (Co-Cr) bare metal stent platform featuring thin struts (80 μm) with a unique two-link open-cell design, which facilitates access to the SB8. In case of ballooning of an SB (e.g., kissing balloon technique [KBT]), the cell could potentially open up to either a 5.3×4.3 mm in 3.5 mm device or a 4.5×3.5 mm in 3.0 mm device (Terumo internal data), suggesting that the Ultimater stent could be available for a large SB (Figure 9). The Ultimaster platform is coated with sirolimus (3.9 μg/mm stent length) and bioresorbable poly (DL-lactide-co-caprolactone) polymer only on the abluminal side. Within three to four months the polymer is either metabolised, through DL-lactide and caprolactone into carbon dioxide and water, or excreted in urine and faeces. Thereafter, the Ultimaster drug-eluting stent (DES) is expected to become a bare metal stent. The Ultimaster DES is available in diameters of 2.25, 2.5, 2.75, 3.0, 3.5 and 4.0 mm, and in length sizes of 12, 15, 18, 24, 28, 33 and 38 mm8,9.
OFDI IMAGING ACQUISITION
The OFDI image catheter should be positioned distally to the target segment. The proximal radiopaque marker of the catheter will be located at approximately >10 mm distally to the distal edge of the stent. Contrast medium will be used for blood removal by a guiding catheter of 6 Fr or greater. Mechanical injection and a pullback speed of 20 mm/sec are recommended.
OFDI CORE LAB ANALYSIS
OFDI recordings will be sent to an independent core laboratory (Cardialysis B.V., Rotterdam, the Netherlands) for assessment. The core laboratory will be blinded to patient allocation. Quantitative analysis will be performed using a dedicated semi-automated contour detection system (QCU-CMS; Medis medical imaging systems bv, Leiden, the Netherlands) using the standard method10,11. The region of interest (ROI) is the stented segment, defined as the region between the first and the last frame in which struts were visible over 360-degree vessel circumference, and 5 mm proximal and distal to the peristent site. The lumen contour is traced automatically for all frames of the ROI with minimal manual corrections if needed. Strut malapposition is defined as a separation of the luminal boundary of one or more of the stent struts from the vessel wall with a distance of more than the strut thickness (80 μm for the Ultimaster stent). The post-procedural percentage of malapposed struts (primary endpoint) will be assessed in the main branch bifurcation region (Figure 3). Any struts without contact with the vessel wall in 2D cross-sections, taking into consideration strut thickness, are considered as malapposed struts. Frame-by-frame cross-sectional images will be analysed for the primary endpoint.
DUAL ANTIPLATELET THERAPY
Dual antiplatelet therapy is continued for at least six months according to the European Society of Cardiology (ESC) guidelines. In acute coronary syndrome (ACS) patients, clopidogrel could be prescribed in case ticagrelor or prasugrel is not available.
FOLLOW-UP
In principle, the study concerns the acute performance of two imaging guidance strategies, and clinical follow-up at six and 12 months post procedure is performed to ensure the safety of the patients by either hospital visit or telephone contact. No angiographic or imaging follow-up is planned. Hospital visits are planned at six months (±14 days) and one year (±30 days).
SAMPLE SIZE CALCULATIONS
Assumptions for sample size determination are based on past registries7,12. A sample size of 106 patients will provide a power of 90% to show superiority of the 3D-OFDI guidance group to the angio-guidance group in the OPTIMUM trial. The assumptions are: 1) the malapposition rate in bifurcation by angio guidance is 26%3, 2) 3D-OFDI guidance reduces malapposition by 50%, 3) the malapposition rate in bifurcation by 3D-OFDI guidance is 13%, 4) the common standard deviation is 20%, 5) a 5% two-sided level of significance (alpha), and 6) 5% insufficient quality of OFDI. To complete the enrolment of 106 patients, the estimated period is two years.
ANALYSIS POPULATION(S)
All clinical data are analysed according to the intention-to-treat (ITT) principle in the ITT population. The ITT population consists of all patients who were randomised, regardless of the actual treatment or per protocol deviations.
Discussion
The feasibility of 3D-OCT-guided PCI in bifurcation has been reported in only a few registries and no prospective randomised trial has been reported. In a registry of 22 patients, Okamura et al demonstrated the investigation of off-line 3D-OCT reconstruction during PCI procedures7,10. 3D-OCT reconstruction was completed in five to six minutes; therefore, the 3D-OCT image was available during the procedure as a “semi-online 3D-OCT”. In this report, Okamura et al emphasised the importance of the configuration of overhanging struts for the incidence of ISA. The percentage of ISA of the free carina type with distal cell rewiring was significantly smaller than that of the cases either of the connecting to carina type or with non-distal cell rewiring (0.7±0.9% vs 12.2±6.5%, p=0.0074). Moreover, Okamura et al recently reported in 105 patients that 1) ISA in the link-free carina configuration and distal re-crossing (LFD) group was significantly lower than that in the non-LFD group (6.7±5.9% vs 17.0±10.5%, p<0.0001), and 2) proximal re-crossing increased ISA compared to distal re-crossing (17.1±10.1% vs 6.3±6.0%, p<0.0001)4. Alegria-Barrero et al12 reported that 52 patients who were treated for bifurcation lesions using OCT-guided re-crossing (n=12) had a significantly lower number of malapposed stent struts (9.5% [7.5-17.4%] in the OCT-guided group vs 42.3% [31.2-54.7%] in the angiography-guided group, p<0.0001). In this study, OCT guidance was based mainly on 2D cross-sections. This study was retrospective, not a randomised trial, not online. When compared with the previous studies, the OPTIMUM trial will be the first prospective, randomised trial to evaluate the efficacy and safety of online 3D-OCT-guided PCI in bifurcation lesions.
Limitations
This is an acute mechanistic study. The clinical impact of ISA at a bifurcation segment (primary endpoint) has not yet been established.
Conclusion
The OPTIMUM trial will be the first prospective, randomised trial to evaluate the efficacy and safety of online 3D-OCT-guided PCI in bifurcation lesions.
|
Impact on daily practice This trial will provide important clinical data on the role of online 3D-OFDI imaging as guidance in bifurcation PCI. Online 3D-OFDI images could help operators to undertake rewiring of the optimal cell, resulting in a lower rate of malapposition compared with angiography-guided PCI. |
Guest Editor
This paper was guest edited by Antonio Colombo, MD; Centro Cuore Columbus, Milan, Italy.
Funding
This study was sponsored by Meditrix, with a grant from Terumo Corporation.
Conflict of interest statement
Y. Onuma has served as a member of the advisory board of Abbott Vascular. Y. Sotomi has received speaker honoraria from Abbott Vascular Japan, Boston Scientific Japan, Terumo, Cardinal Health, and Medtronic. P.W. Serruys reports personal consultancy fees from Abbott Laboratories, Biosensors, Cardialysis, Medtronic, Micell, Sino Medical Sciences Technology, Philips/Volcano, Xeltis, and HeartFlow. The other authors have no conflicts of interest to declare. The Guest Editor has no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Online 3D-OFDI: case example. After the first attempt at re-crossing the wire, online 3D-OFDI detected that the wire re-crossed through a suboptimal cell. After the second attempt at re-crossing the wire, 3D-ODFI showed optimal cell rewiring.
Moving image 2. Online 3D reconstruction using the Terumo OFDI system.

