
Abstract
We present the primary report of a paediatric patient who had placement of the MitraClip device for severe functional mitral regurgitation. The patient was a 14-year-old boy with symptomatic end-stage non-compaction type cardiomyopathy secondary to a mitochondrial cytopathy. He had severe mitral regurgitation, tricuspid valve regurgitation, a severely dilated LV with apical non-compaction, severe LV dysfunction and severe pulmonary hypertension. Despite optimal medical therapy he developed progressive symptoms of congestive heart failure and he was not a candidate for an assist device or cardiac transplantation. Multimodality imaging with fluoroscopy, two-dimensional and three-dimensional transoesophageal echocardiography was used to guide the procedure. Two MitraClips were placed resulting in an adequate decrease in MR severity to no more than mild-moderate. Five months post MitraClip implantation, he has improved exercise tolerance. Transthoracic echocardiography showed mild to moderate mitral regurgitation and a decrease in left ventricular size. To our knowledge, this is the first reported paediatric patient to be offered this innovative procedure. Percutaneous edge-to-edge MV repair may prove to be a novel and effective palliation to consider in a subgroup of paediatric, adolescent and young adult patients. This case report describes some of the considerations specific to the paediatric patient.
Abbreviations
CFD: colour flow Doppler
LAX: long-axis
ME: mid oesophageal
MPR: multiplanar reconstruction
SAX: short-axis
TG: transgastric
Background
Percutaneous edge-to-edge mitral valve (MV) repair performed with the MitraClip® device (Abbott Vascular, Santa Clara, CA, USA) in high-risk or inoperable adult patients with severe symptomatic mitral regurgitation (MR) has been shown to be an effective therapy that reduces symptoms and improves functional status1. The safety and effectiveness of MitraClip therapy has not been established in paediatric patients since, in most instances, other more durable and proven treatment choices such as MV surgery (repair or replacement) or heart transplantation are available to them. Here we present a paediatric patient with heart failure symptoms in conjunction with severe functional MR (FMR) who had successful placement of the MitraClip device.
Case report
The case was a 14-year-old, 50.6 kg (BSA 1.47 m2) boy with severe neurodevelopmental delay and symptomatic end-stage dilated and non-compaction type cardiomyopathy secondary to a mitochondrial cytopathy involving electron transport chain complexes 1 and 4. Despite optimal chronic medical therapy (lisinopril, carvedilol, spironolactone, furosemide) which could not be increased due to hypotension, he developed progressive symptoms of congestive heart failure (CHF).
A pre-procedure transthoracic echocardiogram (TTE) showed a left ventricular ejection fraction (LVEF) of 35-40%, severe central MR, a markedly enlarged left atrium (LA), severe pulmonary hypertension (PHTN) with suprasystemic pulmonary artery pressures (PAP), moderate tricuspid valve regurgitation (TR) and a mild decrease in right ventricular (RV) function. The family declined evaluation for cardiac transplantation but was interested in pursuing percutaneous MV therapy. He was referred for consideration of percutaneous MV repair with the MitraClip device.
Following thorough consultation with all members of the Heart Valve Team and informed consent, percutaneous edge-to-edge repair of his MV was undertaken. A paediatric interventional cardiologist, and paediatric and adult cardiac anaesthesiologist assisted with his care and procedural imaging. A significant amount of preoperative planning was undertaken to care for a child in a hospital that serves adult patients. His unique psychosocial needs as well as the anaesthetic management of his cytopathy were the major differences in his management compared to a typical patient receiving MitraClip therapy.
Multimodality imaging with fluoroscopy and two-dimensional (2D) as well as three-dimensional (3D) transoesophageal echocardiography (TEE) was used to guide the procedure (iE33; Philips Medical Systems, Andover, MA, USA). The pre-procedure TEE exam showed a severely dilated LV with apical non-compaction, severe LV dysfunction and severe MR along the entire coaptation line (Figure 1, Figure 2, Moving image 1, Moving image 2). Based on 3D quantification, the MV annulus was enlarged at 3.7×3.6 cm and the vena contracta area (VCA) measured 0.86 cm2 (Figure 3). 3D colour imaging further elucidated that there were two distinctive MR jets along the coaptation line, a larger medial jet and a smaller more lateral jet (Figure 4, Moving image 3).
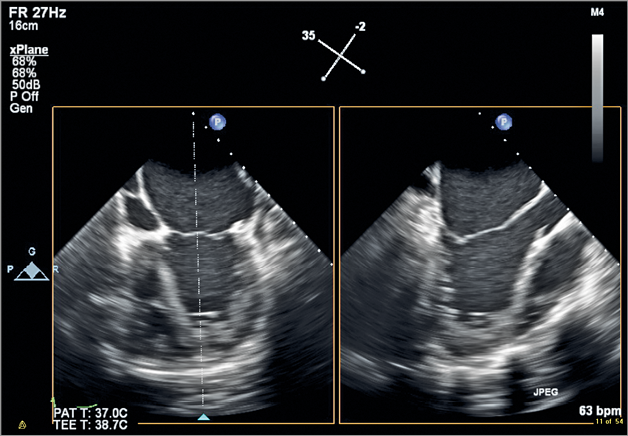
Figure 1. ME biplane acquisition showing a modified four-chamber view on the left and a perpendicular LAX view of the LV on the right. Note the enlarged left atrium and the globular LV with apical non-compaction. The MV leaflets are apically tethered during systole and have little opportunity for coaptation.
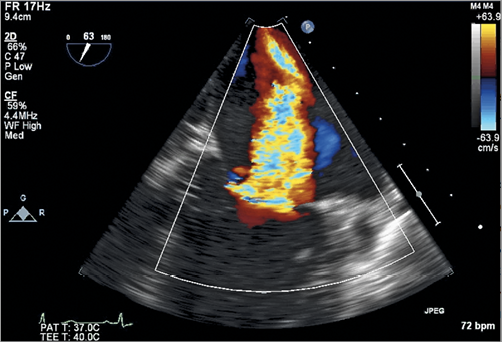
Figure 2. 2D CFD ME commissural view of the MV showing severe MR along the entire coaptation line.
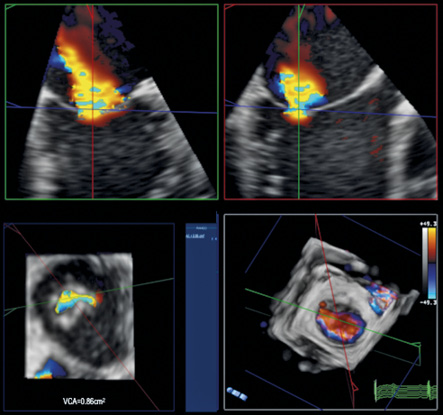
Figure 3. 3D colour MPR of the MR jet before placement of the first MitraClip. The MPRs are accurately aligned to measure the VCA at baseline. Note the elliptical shape of the VCA as typically seen in FMR. At baseline, the VCA measured 0.86 cm2.
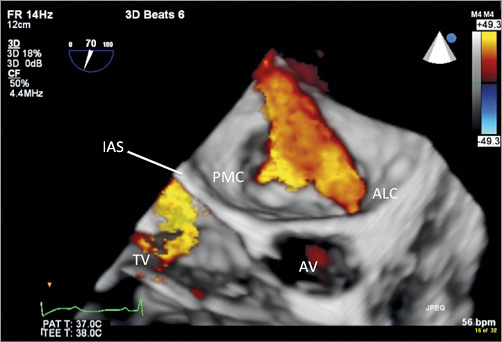
Figure 4. 3D colour image of the MV with the posteromedial commissure (PMC) on the left and the anterolateral commissure (ALC) towards the right. Unidirectional colour selection was used to demonstrate only regurgitant flow while removing the colour distraction created by mitral inflow. Note that, compared to the orthogonal 2D colour images (Figure 2, Moving image2A, Moving image 2B), 3D colour is extremely useful in providing details of the geometry of the MR. This image also shows the aortic valve in front (AV) and moderate TR directed towards the interatrial septum (IAS).
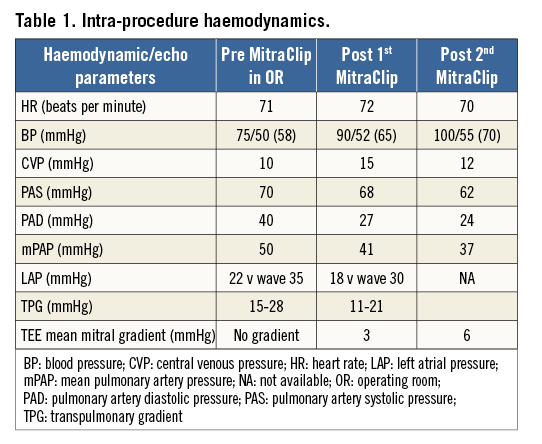
Given the comprehensive echocardiographic assessment at the beginning of the case, the treatment strategy included a high likelihood of requiring two MitraClips to obtain a significant reduction of MR. Thus, the first clip was deployed in the central portion of the MV (A2/P2 segment) with the intention of placing a second clip medial to the first (Figure 5, Moving image 4). The second clip created a much smaller medial orifice and resulted in a further reduction of MR to mild-moderate (Figure 6, Figure 7, Moving image 5) with an increase in the mean gradient across both orifices to 6-7 mmHg. The net result was a slightly greater mean transmitral gradient than desired but, given the significant decrease in MR, there was a net decrease in his PAP (Table 1).
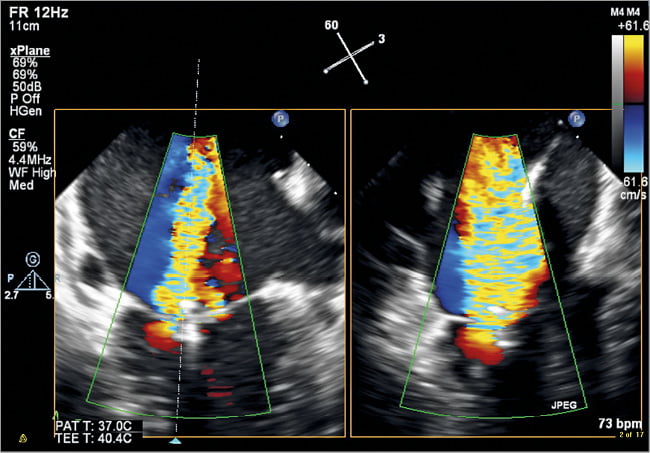
Figure 5. ME CFD biplane image of the MV after deployment of the first MitraClip in the area of A2/P2. MV commissural view on the left and corresponding LAX view of the MV on the right. Note the significant residual MR medial to the first MitraClip.
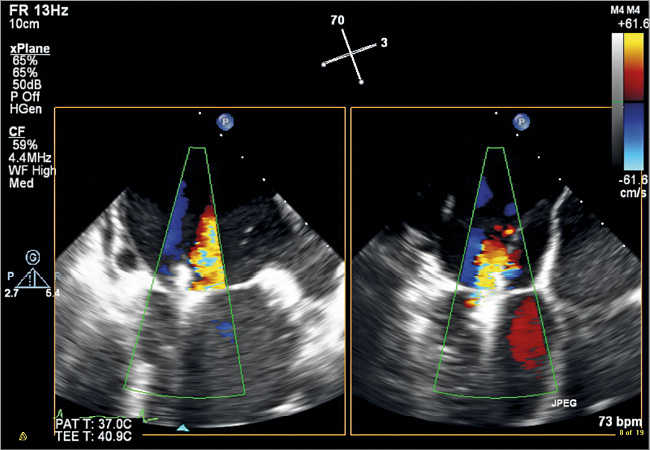
Figure 6. ME colour biplane image displaying the MV in a commissural view and a corresponding LAX view after the second MitraClip has been deployed. The image displays further reduction of MR leaving only mild to moderate residual MR laterally.
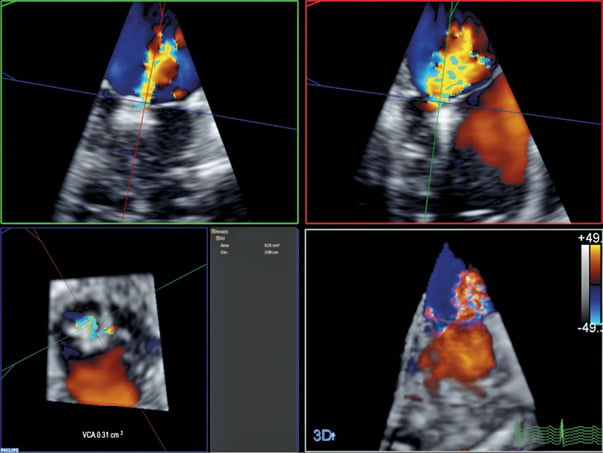
Figure 7. 3D colour MPR of the MR jet following deployment of both MitraClips. The VCA has been significantly reduced to 0.31 cm2 post treatment.
The patient was extubated immediately post procedure and, after a short stay in the recovery unit, was transferred to the paediatric intensive care unit at Seattle Children’s Hospital. He was discharged from hospital four days later.
On his recent follow-up visit, five months post MitraClip therapy, the patient was in satisfactory condition. His TTE showed the two clips in stable position. Compared to the pre-procedure TTE, there was moderate MR, a decrease in left ventricular internal diameter in diastole to 52 mm from 59.3 mm, a decrease in LA volume index to 62 ml/m2 from 72.2 ml/m2, an improvement in PAP to ¾ of systemic pressure and an increase in LVEF from 39% to 41%. The family described improved exercise tolerance and appetite. His brain natriuretic peptide (BNP) is trending downwards.
Discussion
Paediatric patients with cardiomyopathies secondary to genetic or congenital diseases, such as mitochondrial cytopathies, muscular dystrophies and other metabolic disorders, can present with a wide range of clinical symptoms and prognoses. At the severe end of the spectrum, they may have associated significant comorbidities such as respiratory failure, severe neurocognitive or neuromuscular dysfunction, resulting in limited life expectancy2. Once these patients develop symptoms of advanced CHF, few treatment options are offered beyond medical therapy. Mechanical support and heart transplantation are often not considered because life expectancy remains limited by non-cardiac comorbidities.
Percutaneous edge-to-edge MV repair decreases FMR, LV dimensions, and improves symptoms in adults who are not candidates for surgical repair and may prove to be a novel and effective palliation to consider in paediatric patients with limited therapeutic options.
For technical reasons, the patient’s cardiovascular anatomy (especially peripheral vessels) needs to be large enough to accommodate the 24 Fr introducer sheaths as well as allowing manoeuvrability of the MitraClip delivery system within the left atrium. These requirements correspond to a minimum weight of approximately 40-45 kg and minimum age of about nine to 12 years. The procedure is performed using the same size transseptal puncture needle, MitraClip and percutaneous closure devices as in adults.
Although there is the concern for an increased risk of creating mitral stenosis in smaller patients, most patients with FMR have a dilated MV annulus and increased LA volumes. In addition, even at the lower age of this range, the annulus is usually more than 85% of its expected adult size3. Finally, there are reports describing surgically placed Alfieri stitches in paediatric patients as young as two years without creating mitral stenosis4. Therefore, it seems as though the risk of creating unacceptable mitral stenosis may be less than anticipated based on age.
Conclusion
To our knowledge, this is the first reported paediatric patient to be offered this innovative procedure for worsening FMR in the setting of a cardiomyopathy. Preliminary results from early follow-up indicate that percutaneous edge-to-edge MV repair may have a role as compassionate therapy in a subgroup of paediatric, adolescent and young adult patients with advanced FMR.
| Impact on daily practice This is a primary report on the use of the MitraClip procedure to palliate symptomatic severe functional MR in a paediatric patient with a limited life expectancy. Although the MitraClip procedure was not conceptualised for the paediatric population, this treatment option should be considered as compassionate therapy in a subgroup of paediatric, adolescent and young adult patients with advanced FMR. |
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. 2D TEE biplane image of the left ventricle. ME biplane acquisition showing a modified four-chamber view on the left and a perpendicular LAX view of the LV on the right. Note the enlarged left atrium and the globular LV with apical non-compaction. The MV leaflets are apically tethered during systole and have little opportunity for coaptation. There is posterior and lateral wall akinesis.
Moving image 2. 2D TEE colour flow Doppler of the MV. 2D CFD ME commissural view of the MV showing severe MR originating along the entire coaptation line.
Moving image 3. 3D colour moving image of the MV with the posteromedial commissure (PMC) on the left and the anterolateral commissure (ALC) towards the right. Unidirectional colour selection was used to demonstrate only regurgitant flow while removing the colour distraction created by mitral inflow. Note that, compared to the orthogonal 2D colour images (Figure2, Moving image2), 3D colour is extremely useful in providing details of the geometry of the MR. Two distinct jets are clearly seen: a large medial component and a smaller lateral one. This moving image also shows the aortic valve in front (AV) and moderate TR directed towards the interatrial septum (IAS).
Moving image 4. ME CFD biplane image of the MV after deployment of the first MitraClip in the area of A2/P2. The commissural view is seen on the left and a corresponding LAX view of the MV on the right. Note the significant residual MR medial to the first MitraClip.
Moving image 5. ME colour biplane video displaying the MV in a commissural view and a corresponding LAX view after the second MitraClip has been deployed. The moving image displays further reduction of MR leaving only mild to moderate residual MR laterally.
Supplementary data
To read the full content of this article, please download the PDF.
2D TEE biplane image of the left ventricle. ME biplane acquisition showing a modified four-chamber view on the left and a perpendicular LAX view of the LV on the right. Note the enlarged left atrium and the globular LV with apical non-compaction. The MV leaflets are apically tethered during systole and have little opportunity for coaptation. There is posterior and lateral wall akinesis.
2D TEE colour flow Doppler of the MV. 2D CFD ME commissural view of the MV showing severe MR originating along the entire coaptation line.
3D colour moving image of the MV with the posteromedial commissure (PMC) on the left and the anterolateral commissure (ALC) towards the right. Unidirectional colour selection was used to demonstrate only regurgitant flow while removing the colour distraction created by mitral inflow. Note that, compared to the orthogonal 2D colour images (Figure 2, ), 3D colour is extremely useful in providing details of the geometry of the MR. Two distinct jets are clearly seen: a large medial component and a smaller lateral one. This moving image also shows the aortic valve in front (AV) and moderate TR directed towards the interatrial septum (IAS).
ME CFD biplane image of the MV after deployment of the first MitraClip in the area of A2/P2. The commissural view is seen on the left and a corresponding LAX view of the MV on the right. Note the significant residual MR medial to the first MitraClip.
ME colour biplane video displaying the MV in a commissural view and a corresponding LAX view after the second MitraClip has been deployed. The moving image displays further reduction of MR leaving only mild to moderate residual MR laterally.

