
Introduction
Rotational atherectomy (RA) is the most commonly used technique to modify calcific coronary artery disease (CAD) to facilitate stent implantation and deployment. While embolisation of fragmented plaque into the microcirculation during RA is thought to be associated with a higher incidence of procedural myocardial infarction (type 4a MI), this has never been comprehensively demonstrated1,2.
The definition of type 4a MI is contentious with two definitions, the third universal definition (3rd UD) and the Society for Cardiovascular Angiography and Interventions (SCAI) definition, currently widely used3,4. The SCAI definition has been used in recent clinical trials aiming to capture more prognostically important infarcts.
With the increasing prevalence of calcific CAD there is a need to determine the safety of adjunctive interventional techniques, such as RA. The aim of this study was to determine the incidence of type 4a MI following percutaneous coronary intervention (PCI) with RA and investigate the associated cardiac magnetic resonance (CMR)-detected injury. ClinicalTrials.gov Identifier: NCT02857790.
Methods
This was a prospective cohort study conducted at a regional heart centre in the United Kingdom. We recruited 58 near-consecutive, eligible patients with stable angina requiring PCI with RA to a calcific coronary lesion.
The exclusion criteria were: MI within prior seven days; Q-waves on ECG, wall motion abnormality (WMA) on echocardiogram, or infarct on baseline CMR in target vessel territory; contraindication to CMR; chronic total occlusion (CTO), and eGFR <30 ml/min/1.73 m2.
Multi-parametric CMR was performed before RA, seven days post RA, and six months post RA. The protocol included T2 mapping for myocardial oedema, cine CMR for regional WMA, rest first-pass and adenosine stress perfusion, and late gadolinium enhancement (LGE) for scar. Image analysis was performed by blinded analysts (G. Clerfond, D. Corcoran, C. Berry) (QMass®; Medis, Leiden, the Netherlands).
The CMR diagnosis of an MI was based on rest first-pass perfusion, cine WMA, and LGE. A new MI was diagnosed if LGE was confirmed on both axial and long-axis acquisitions, or a new WMA was detected.
For PCI, patients received dual antiplatelet therapy and unfractionated heparin (target activated clotting time [ACT] >250 seconds). RA was performed with continuous heparinised saline flush in 20-second runs at 160,000 rpm (if required increasing to a maximum of 200,000 rpm) until the lesion was modified (ROTALink™ System; Boston Scientific, Marlborough, MA, USA). Burr size and the use of multiple burrs were at the operator’s discretion. Lesions were predilated and post-dilated with non-compliant balloons, and drug-eluting stents implanted. Intravascular ultrasound (IVUS) was used when technically possible (OptiCross™ Coronary Imaging Catheter; Boston Scientific). High-sensitivity troponin (Tn) was measured at six to 18 hours post procedure.
We determined the incidence of type 4a MI using the 3rd UD (Tn >5x 99th percentile URL within 48 hours of procedure, plus either (i) prolonged chest pain [>20 min], (ii) ischaemic ST changes or new pathological Q-waves, (iii) angiographic evidence of a flow-limiting complication, or (iv) imaging evidence of new loss of viable myocardium or new WMA)3, and the SCAI definition (CK-MB ≥10 ULN or Tn ≥70 ULN [or CK-MB ≥5 ULN or Tn ≥35 ULN plus new Q-waves in ≥2 contiguous leads or LBBB])4.
Results
Major adverse cardiovascular cerebrovascular events (MACCE) and CCS angina grade were recorded.
The study CONSORT diagram is shown in Figure 1. Baseline demographics, procedural characteristics, and QCA analysis are reported in Table 1-Table 3. CMR and angiographic images of an example study patient are illustrated in Figure 2.
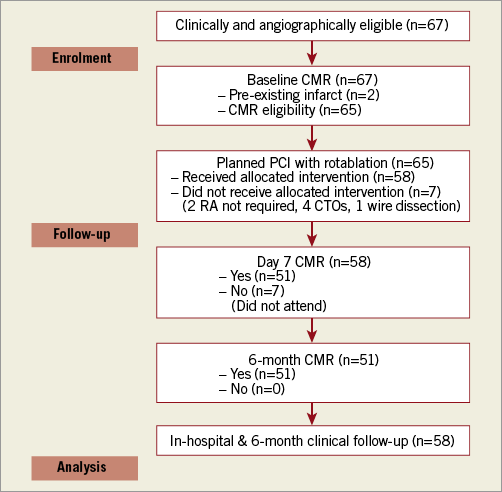
Figure 1. CONSORT diagram.
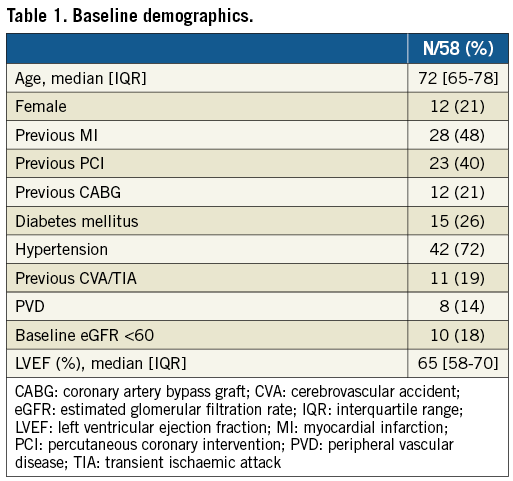
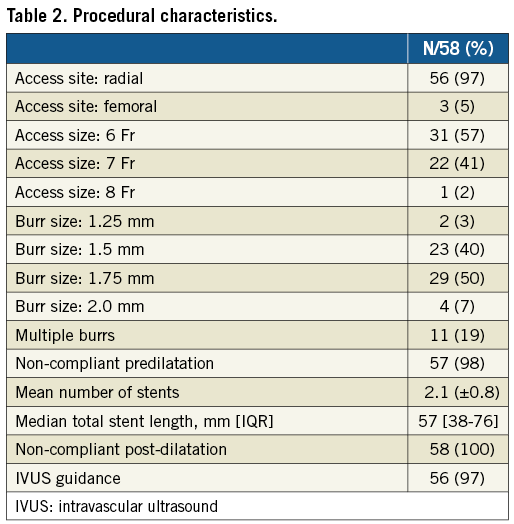
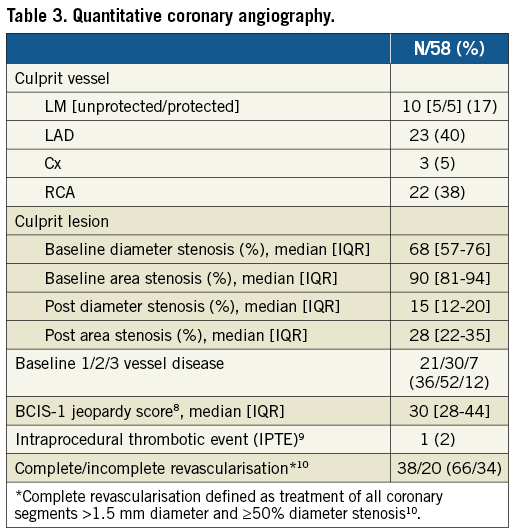
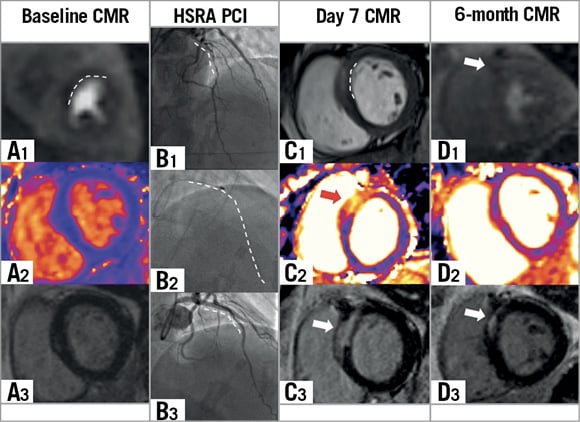
Figure 2. CMR and angiographic images of a study patient. A 44-year-old male with angina and calcific proximal LAD disease. PCI with RA with no events or symptoms in-hospital or at six months. A1) Baseline adenosine stress perfusion short-axis image demonstrating an anteroseptal perfusion defect. A2) Baseline T2 map demonstrating no myocardial oedema. A3) Baseline LGE image demonstrating no prior LGE. B1) Baseline angiogram demonstrating calcific LAD lesion. B2) Angiogram demonstrating 1.5 mm rotablator burr. B3) Final angiogram following stent implantation. C1) Day 7 short-axis cine image demonstrating basal anteroseptal oedema and WMA. C2) Day 7 T2 map demonstrating basal anteroseptal oedema. C3) Day 7 LGE demonstrating new basal anteroseptal LGE. D1) Six-month adenosine stress perfusion short-axis image demonstrating small residual anteroseptal perfusion defect. D2) Six-month T2 map demonstrating resolution of basal anteroseptal oedema. D3) Six-month LGE demonstrating persistent basal anteroseptal scar. CMR: cardiac magnetic resonance; HSRA: high-speed rotational atherectomy; LAD: left anterior descending artery; LGE: late gadolinium enhancement; PCI: percutaneous coronary intervention; WMA: wall motion abnormality
A 3rd UD type 4a MI occurred in 12 (24%) patients (Table 4). If this was determined without CMR imaging (as performed in routine clinical practice), the incidence was 10% (N=5), and using the SCAI definition 4% (N=2).
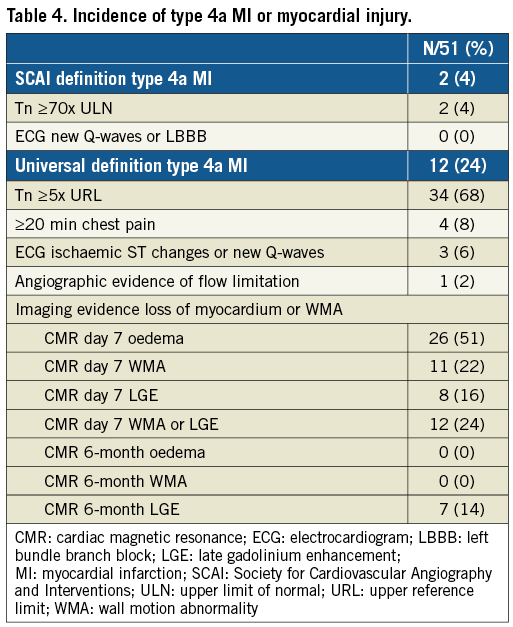
On day 7 CMR, 26 patients (51%) had myocardial oedema, 11 (22%) new WMA, and 8 (16%) new LGE. At six months, all oedema and WMA had resolved but 7 patients (14%) had persistent LGE.
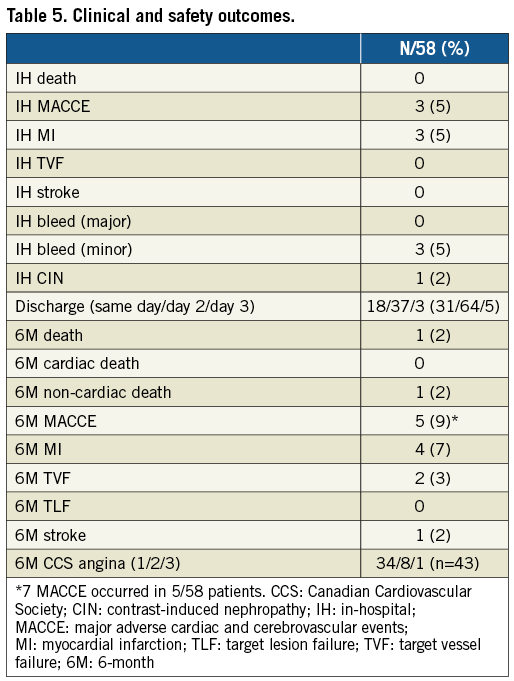
There was a significant reduction in the number of ischaemic segments from baseline, at day 7 and six months (5 [3-7] vs. 2 [0-3] vs. 0 [0-2], p<0.001), and 79% of patients had CCS 1 angina (Table 5). There were seven MACCE in 5/58 patients (9%) at six months (Table 5).
Patients who had a 3rd UD type 4a MI were older (mean age 82 vs. 69 years), more commonly female (33% vs. 19%), diabetic (42% vs. 23%), and less often had a prior CABG (8% vs. 23%), compared to those without an MI.
Discussion
To our knowledge, this is the first study to investigate specifically the incidence of procedural MI following PCI with RA. The diagnosis was systematically determined using high-sensitivity Tn, clinical symptoms, ECG changes and CMR, and in doing so detected 3rd UD type 4a MI in 24% of patients. If the CMR imaging was not used (10%) or the SCAI definition applied (4%), the incidence was significantly lower. The further observation that all early oedema and WMAs resolved at six months, while LGE persisted, highlights the importance of differentiating between transient myocardial injury and clinically significant infarction.
In comparison to our data, in a large prospective cohort of “all-comer” PCI patients (with no CMR) 3rd UD type 4a MI was also reported in 10%2. In a small cohort of patients having multivessel PCI, 82% had a Tn >3x URL5, comparable to 84% in our cohort. In CTO PCI, 11% of patients had CK-MB >3x URL6. Accepting Tn >40x URL equates to CK-MB >3x URL5, this would compare to an incidence of 6% in our cohort.
While large infarcts predict mortality, all type 4a MIs are not associated with long-term outcomes1. A patient-level pooled analysis of NSTEMI trials found that procedural MI was not associated with increased cardiovascular death at five years7. Furthermore, the five-year cardiovascular death rate was 22% and 5% in patients with a further spontaneous MI compared to those with a procedural MI. This differentiates between ischaemia in a controlled environment with immediate treatment compared to a spontaneous event associated with a more prolonged and systemic response.
The sensitivity and specificity of the 3rd UD and SCAI definition to detect LGE were 100% and 90% versus 25% and 100%, respectively. If residual scar is associated with arrhythmic risk (as is being investigated in the CMR-GUIDE study, NCT01918215), our data would support the use of the 3rd UD.
Limitations
While this is a small cohort with a large burden of calcific disease, it is the largest series of patients having PCI with RA with serial CMR published to date. This was a single-centre study, a design determined by the complexity of the population and protocol. While noting the high prevalence of ≥1.75 mm burrs, these patients had fewer 3rd UD type 4a MIs (19% vs. 26%) and less CMR-detected LGE (7% vs. 21%) than those treated with ≤1.5 mm burrs. Without a control group, and treatment with long-stented segments and non-compliant balloons, we cannot conclude that myocardial injury was exclusively related to RA. While seven of 58 patients did not complete all CMR time points, this was not unexpected in older patients with multiple comorbidities. Longer-term clinical follow-up would be of increased value.
Conclusions
PCI with RA was associated with a significant risk of type 4a MI, although myocardial injury had resolved in most patients at six months. This was paralleled by a sustained reduction in ischaemia and angina burden.
| Impact on daily practice These data will impact on patient consent for these complex interventions, informing discussion around the trade-off between the risk of transient ischaemic injury or persistent scar, versus symptomatic benefits and reassuring midterm outcomes. |
Acknowledgements
We would like to acknowledge the support of our staff in delivering this project. Siemens Healthcare provided work-in-progress imaging methods.
Funding
This work was supported by the Chief Scientist Office, Medical Research Scotland, Scottish Funding Council, Engineering and Physical Sciences Research Council (EP/N014642/1) and the British Heart Foundation (BHF CoRE – RE/13/5/30177; BHF Clinical Research Training Fellowship – D Corcoran: FS/14/15/30661).
Conflict of interest statement
C. Berry and K. Oldroyd have acted as consultants to St. Jude Medical. These companies had no involvement in this research or manuscript. The other authors have no conflicts of interest to declare.

