
CASE SUMMARY
BACKGROUND: A 68-year-old man with a history of prior endovascular abdominal aortic aneurysm repair, with recurrent flash pulmonary oedema and renal artery revascularisation including redo renal artery interventions for in-stent restenosis, presented to our department with renal failure, severe uncontrolled high blood pressure and left renal stent fracture. The patient was prepared for a third renal revascularisation procedure.
INVESTIGATION: Renal angiography, intravascular ultrasound, renal duplex imaging
DIAGNOSIS: Renal artery in-stent restenosis and stent fracture after endovascular abdominal aortic aneurysm repair.
MANAGEMENT: Left renal artery stenting was performed using a Hippocampus 6×24 mm renal stent which was preferred to a drug-eluting stent.
KEYWORDS: abdominal aortic aneurysm repair, complication, drug-eluting stent, renal artery in-stent restenosis, renal stent, stent fracture
PRESENTATION OF THE CASE
A 67-year-old gentleman with hypertension was admitted to the clinic five months after endovascular abdominal aortic aneurysm repair (EVAR), with flash pulmonary oedema (FPE) and renal failure (serum creatinine of 4.3 mg/dl). An Endurant bifurcated stent graft (Medtronic, Minneapolis, MN, USA) with right iliac extension had been previously implanted at another clinic.
Previous medical records and pre- and post-procedure CT imaging from the department where EVAR was performed revealed no renal artery stenosis or diaphragm anomalies. After intensive treatment with diuretics, beta-blockers and calcium antagonists, the patient was stabilised and the renal function improved to a creatinine level of 1.8 mg/dl. No ACE inhibitors were administered. Using a right radial approach, renal angiography showed bilateral high-degree renal artery stenosis (Figure 1). Both renal arteries were stented using Herculink stents (RX Herculink Elite® Stent System; Abbott Vascular, Santa Clara, CA, USA), 5×18 mm for the right renal and 6×18 mm for the left renal artery (Figure 2). Three days later, the patient was discharged, having completely recovered with a creatinine level of 1.55 mg/dl, normal blood pressure on beta-blockers, calcium blockers, ACE inhibitors, aspirin and clopidogrel.
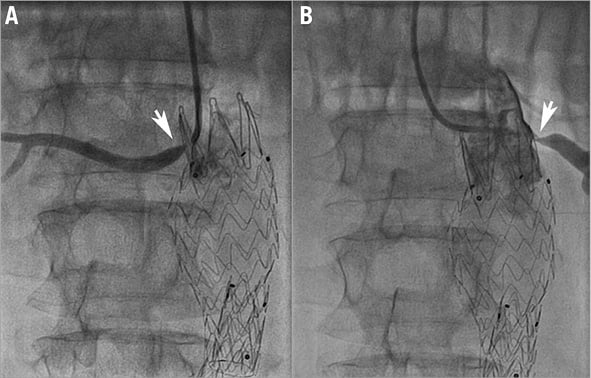
Figure 1. Selective right and left renal artery angiography showing bilateral severe ostial stenosis (arrows). A) Right renal artery angiography. B) Left renal artery angiography.
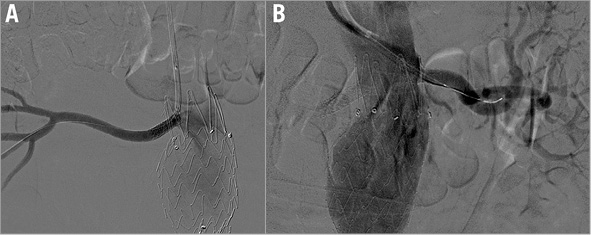
Figure 2. Renal angiography after bilateral renal artery stenting. A) Right renal artery angiography. B) Left renal artery angiography.
One year later, the patient was readmitted for FPE and renal failure. Renal angiography showed severe bilateral in-stent restenosis (Figure 3A-Figure 3C). A 4×23 mm XIENCE V® Everolimus Eluting Coronary Stent (Abbott Vascular) was implanted in the right renal artery and post-dilatation was performed with a 5×20 mm balloon with a good result (Figure 3B). The angioplasty was performed using the left radial approach as a bail-out procedure with the intention of resolving the critical condition of FPE. However, left renal artery revascularisation with a drug-eluting balloon (DEB) was scheduled as well. The procedure was postponed because the DEB was unavailable at that time. The two-month admission showed that the patient was in relatively good clinical condition, but with poorly controlled hypertension on four antihypertensive agents and chronic kidney disease (serum creatinine 1.9 mg/dl, estimated glomerular filtration rate 42 ml/min/1.73 m2).
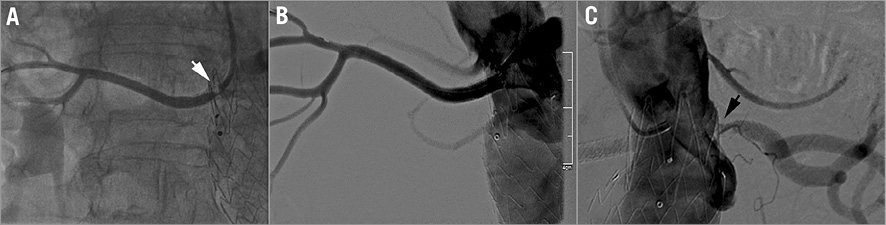
Figure 3. Angiography revealing severe bilateral renal artery restenosis, followed by ostial right renal artery DES implantation. A) Severe in-stent restenosis of the right renal artery (white arrow). B) Right renal angiogram after drug-eluting stent implantation. C) Severe ostial stenosis of the left renal artery.
Redo angiography of the right renal artery showed 18% stenosis at the ostium, which was not present initially (Figure 4A). For this new issue, intravascular ultrasound (IVUS) was used to define the drug-eluting stent (DES) morphology in the right renal artery better. IVUS revealed good apposition of the two stents except for recoil of the DES corresponding with the angiographic 18% stenosis; however, no evidence of restenosis was seen (Figure 4B, Figure 4C). Angiography of the left renal artery revealed a tight ostial restenosis, confirmed by IVUS, with lumen loss caused by a large neointimal tissue proliferation with no stent fracture (Figure 5A, Figure 5B). A 6×20 mm FREEWAY™ 035 DEB (Eurocor GmbH, Bonn, Germany) was used to dilate the left renal stent and a good result was achieved (Figure 5C). This time a right femoral approach was used with the aim of advancing the DEB through an 8 Fr guiding catheter as is recommended by the manufacturer. The patient was discharged with normal blood pressure on triple drug therapy and a creatinine level of 1.2 mg/dl.
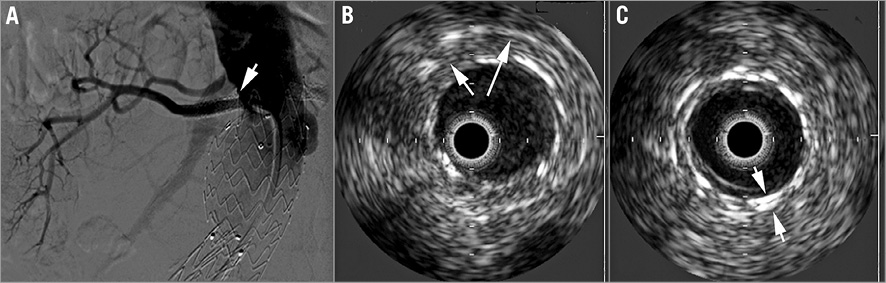
Figure 4. Right renal artery angiography and IVUS imaging. A) Right renal artery angiogram after DES implantation. Note the 18% stenosis at the origin (white arrow). B) IVUS imaging of the proximal right renal artery with recoil of the DES, corresponding to the mild angiographic ostial stenosis. C) Good apposition of the two stents in the middle part of the vessel.
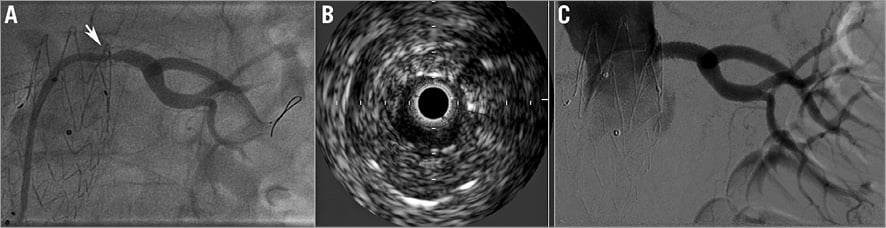
Figure 5. Left renal artery angiography and IVUS. A) Angiography of the left renal artery showing severe in-stent restenosis. B) IVUS of the left renal artery showing severe in-stent restenosis. C) Angiography after DEB angioplasty.
On maximal drug therapy with three drugs the patient’s blood pressure was 170/100 mmHg with a serum creatinine level of 1.78 mg/dl (estimated glomerular filtration rate of 45 ml/min) at the eight-month follow-up. Renal duplex imaging showed normal velocities in the right renal artery but significant recurrent restenosis was observed on the left side (400 cm/sec systolic velocities). The right kidney long axis measured 11.5 cm and the left measured 10.5 cm. Renal artery angiography revealed an unchanged angiographic aspect of the right renal artery but significant in-stent restenosis of the left renal artery. A type I stent fracture of the left renal stent was noted, with phasic angulation of the left renal artery at the fracture site (Figure 6, Moving image 1). These modifications were evident during respiratory motions. The patient was considered for a third revascularisation procedure.
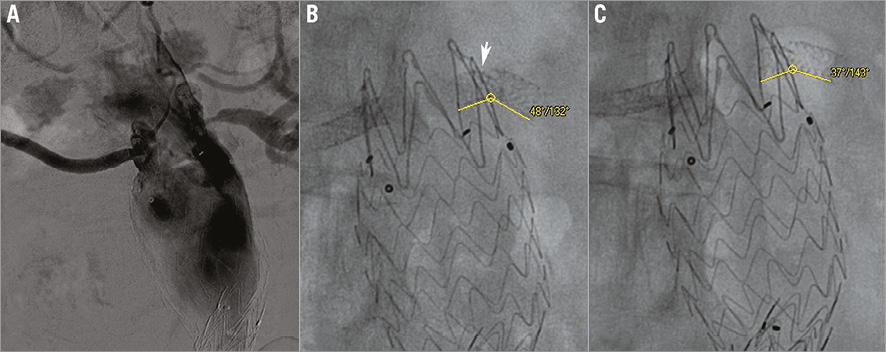
Figure 6. Severe ostial left renal artery re-restenosis and stent fracture. A) Angiography showing tight left renal artery in-stent iterative restenosis. B) The fractured stent parts create an acute angulation with expiration (132 degrees) (arrow). C) Somewhat lesser angulation with inspiration (143 degrees).
How would I treat?
THE INVITED EXPERTS’ OPINION

The patient was treated with EVAR using suprarenal fixation for an aneurysm with a very short neck. Following this, both previously healthy renal arteries developed symptomatic, critical stenosis and were treated with renal BMS. Subsequently, both arteries developed in-stent restenosis. The right renal artery was treated with a DES showing a favourable follow-up. The left renal artery was treated with a DEB followed by a second restenosis and late fracture of the BMS, as shown by IVUS.
There are two problems in this case, the first being the mechanical impact of the stent graft on the renal arteries, and the second being restenosis of the renal stents, which is reported in up to 23% of cases1.
1) In this patient extreme mechanical forces are working on the renal stent: ostial aortic lesions vs. aortic stent graft (possibly migrating because of the short neck) vs. respiratory movements. These forces caused in-stent restenosis and stent fracture. To overcome this problem, the renal stents need to have a good radial force and need to be positioned at least 2-3 mm into the aortic lumen, as correctly performed in this patient (Figure 2). The BMS used in the first place (Herculink; Abbott Vascular, Santa Clara, CA, USA) is a specifically designed renal stent with a reasonable radial force, but, as shown here, it may fracture. Implantation of a second stent definitely increases radial force and worked well in the right renal artery of this patient.
2) To overcome the problem of restenosis, renal arteries really require an antiproliferative drug. Unfortunately, the radial force of a coronary DES may not be sufficient for renal arteries. Alternatively, the still available, first-generation and thick-strut TAXUS™ 5 mm diameter stent (Boston Scientific, Marlborough, MA, USA) or an elective two-stent strategy (DES in BMS or DES in DES) may be used. In this specific patient, the head-to-head comparison of DES vs. DEB for the treatment of in-stent restenosis was clearly in favour of DES. So far we do not have enough data confirming the effectiveness of DEB for renal artery in-stent restenosis. In large vessels such as renal arteries (≥5 mm in diameter), an additional layer of DES appears feasible, increases radial force, and guarantees the delivery of an antiproliferative drug to the vessel.
Another possibility to treat renal artery in-stent restenosis is balloon-expandable covered stents2,3. In our experience, the relatively low-profile Advanta™ V12 covered stent (Atrium, Hudson, NH, USA) has shown good radial force with a low incidence of restenosis. The risk of stent thrombosis, however, needs to be considered as being higher for a covered stent compared to BMS or DES.
Before any treatment, the presence of an endoleak needs to be excluded. The angiography shown in Figure 3C seems a little suspicious. In case of an endoleak, the stent graft needs to be fixed with a proximal cuff, and two covered stents should be implanted in the renal arteries according to the chimney technique3.
To cut a long story short, treatment of the left renal artery with an additional in-stent DES would be our first choice. In case of further events, a covered stent may be considered and, if there is an endoleak, then use the chimney technique.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How would I treat?
THE INVITED EXPERTS’ OPINION

The authors report the case of a recurrent bilateral renal in-stent restenosis (ISR) in a patient previously treated with EVAR. In spite of a good result on the right side after second stent deployment, the authors decided to treat left renal artery ISR with a drug-coated balloon (DCB), without success, since the patient came back with ISR and stent fracture.
The case presented depicts a challenging and increasingly common scenario. Renal artery stenting has been increasingly used for snorkel procedures in aneurysmal diseases. Studies evaluating renal artery motion in patients with various endograft configurations have demonstrated that graft repairs with renal stents reduce motion to 25% of the preoperative value4. Data from computational fluid dynamic models suggest that the vascular geometry created by a stent causes local alterations in wall shear stress, possibly determining intimal hyperplasia and subsequent restenosis5. Being related to these mechanical issues, renal ISR hardly benefits from DCB treatment. In this case, we would focus on a number of technical issues:
1) Was stent positioning good during the first procedure? It is known that in EVAR patients a stent not covering the ostium or protruding too much into the aorta is subject to mechanical stress which increases the risk of fracture.
2) IVUS during the second and third attempts should clarify the relation between stent struts and aortic graft. (Was there any compression? Motion images would be interesting).
3) Stent fracture is probably the result of the mechanical stress occurring during respiration at the stented sites: a double layer of stents may have protected the right side, while DCB treatment was not effective at all in preventing restenosis on the left side (the left renal artery angle is often a risk factor). After second restenosis, we would suggest deploying a 5.0 mm coronary drug-eluting stent inside the previous one and checking with IVUS for proper strut crossing and stent protrusion beyond the stent graft “free flow”; post-dilation with a 6.0 mm balloon would be mandatory. Although an antiproliferative drug could help in preventing restenosis, and a second layer of metallic struts should increase radial strength, mechanical forces would still be an issue. Consequently, a strong recommendation to avoid deep respiratory variations (such as those occurring in patients with episodes of apnoea) should be provided, especially in the presence of a stent on the left renal artery6. Finally, we would suggest (if not yet done) ruling out obstructive sleep apnoea syndrome or, if confirmed, treating it.
Conflict of interest statement
The authors have no conflicts of interest to declare.
How did I treat?
ACTUAL TREATMENT AND MANAGEMENT OF THE CASE
Instead of a DES, a Hippocampus 6×24 mm renal stent (Invatec, Roncadelle, Italy) was chosen and the left renal artery was stented (Figure 7). It was considered that 6 mm overexpansion of a 4 mm DES (the maximum diameter available) would negatively affect the radial force in such a demanding mechanical situation. This was the case with the right renal artery “sandwich” stent , which suffered a late lumen loss due to ostial recoil (Figure 4). After the procedure, the phasic angulation of the left renal artery clearly diminished and no residual stenosis was seen (Moving image 2). Three days after the procedure, the patient had a creatinine level of 1.2 mg/dl and normal blood pressure on triple drug treatment. Dual antiplatelet therapy (clopidogrel 75 mg plus aspirin 100 mg) was administered for one month. Six months after the last renal stenting, following the antihypertensive treatment, the creatinine level and blood pressure were in the normal range. Also, renal duplex imaging showed normal velocities in the left renal artery.
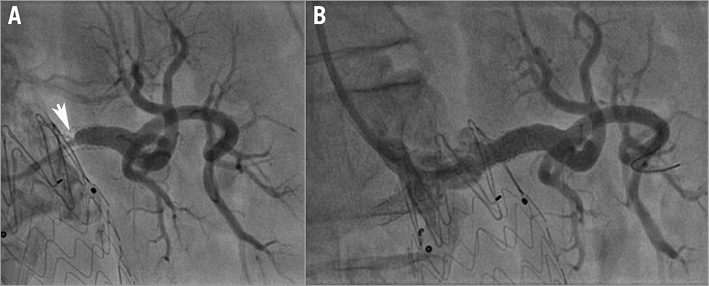
Figure 7. Renal stent implantation on the left renal artery re-restenosis. A) Angiographic aspect of the left renal artery before Hippocampus renal stent implantation. B) Angiographic aspect of the left renal artery after Hippocampus renal stent implantation.
Discussion
FPE is a life-threatening clinical condition secondary to severe renovascular disease. By consensus, FPE is accepted as one of the clearest indications for considering renal artery revascularisation7. During EVAR, suprarenal endograft fixation is sometimes required. Positioning of struts across the renal ostia has been used to prevent stent graft migration and to decrease the rate of complications. This appears to have a minimal effect on renal artery flow rates8,9. Furthermore, some investigators found no significant alteration in the ostial morphology due to the suprarenal struts10. Progressive deterioration in renal function after EVAR can occur over time. Its aetiology is unclear: it is probable that embolisation, contrast media and graft misplacement may play a role. In this case, the mechanism of FPE and renal failure was secondary to bilateral unexpected ostial stenosis.
The early renal artery ostial stenosis might be secondary to altered mechanical forces and aneurysm remodelling, the main body of the stent graft being positioned clearly distal of the renal artery origins. All these can cause dislodgement of atheromatous material from the aneurysmal neck or plaque shift11. A remodelling process of the abdominal aorta after EVAR could also be involved in the occurrence of in-stent restenosis, although restenosis in renal stents is not uncommon. Because of the well-known higher rate of in-stent restenosis after plain balloon angioplasty of the renal artery12,13, drug-eluting balloon therapy was chosen. The occurrence of renal artery stent fracture was unexpected, making the treatment more controversial. Some authors have suggested renal artery entrapment by the diaphragmatic musculotendinous fibres, and a mobile kidney with an acute angulation at the proximal segment of the renal artery, as being the two mechanisms for stent fracture14.
In the present case, the CT scan did not demonstrate a compression of the stent by the left crus of the diaphragm. Our evaluation (Moving image 1) clearly demonstrated phasic respiratory mobility of both renal arteries with larger deformation of the left renal artery stent at its mid segment as being the mechanism of stent fracture.
In one study, stent fractures with short-necked and juxtarenal abdominal aortic aneurysm with fenestrated stent grafts were observed to be related to less than optimal apposition between fenestration and the target vessel ostium15. We did not find any study regarding this issue of stent fracture in non-fenestrated EVAR.
However, in a recent study on patients with abdominal aortic aneurysms, both renal arteries exhibited additional deformations during respiration16. On the other hand, the endovascular stent graft appears to change the renal artery movement by limiting motion of the proximal renal artery motion, while the motion of the distal part remains unaffected17.
It can be speculated that all these new conditions could be involved in “metallic fatigue”, and therefore in stent fracture. Right renal artery DES revascularisation was a viable therapeutic option for this patient with such an urgent and recurrent scenario, knowing that 4 mm stents can be safely expanded up to 5 mm18.
The “sandwich stents” of the right renal artery had a good long-term patency, but some recoil occurred in the critical ostial area. DES recoil was an important issue for the treatment options for the left renal restenosis and stent fracture.
A second DES for the left renal artery could have been a good solution to prevent neointimal proliferation but not for good scaffolding. Foin et al18 have shown that a 4 mm diameter DES can be safely overexpanded to 5 mm, but excessive overexpansion leaves large gaps between the rings that may affect the ability of the stent to scaffold. For this reason and the presence of mechanical challenge on the left renal artery, a 6×24 mm Hippocampus renal artery stent was used. It is well known that dedicated renal stents have better radial forces compared with coronary stents. For this reason, for renal arteries larger than 5 mm they are a better solution than overexpanded coronary DES.
In-stent restenosis remains one of the most important issues after renal stenting with bare metal stents. Methods of treating renal artery in-stent restenosis include simple balloon angioplasty, cutting balloon angioplasty, vascular brachytherapy, repeat bare metal stenting, placement of drug-eluting stents, drug-eluting balloons, stent graft and surgical bypass. Historically, the most common method of treating in-stent restenosis, angioplasty, has been associated with a high re-restenosis rate13. The evidence behind the use of coaxial stent placement in these mechanically challenging conditions is limited, and there are no randomised clinical trials comparing it to angioplasty alone or to the more sophisticated DEB. Renal stent grafts are often used in aortic fenestrated stent grafts and have been associated with less fracture than uncovered stents19. However, we did not find any trial comparing the use of covered stents versus non-covered ones in renal in-stent restenosis associated with stent fracture.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
Moving image 1. Phasic respiratory mobility of both renal arteries with a larger deformation of the left renal artery stent at its mid segment, consistent with stent fracture.
Moving image 2. Disappearance of phasic angulation of the left renal artery.
Supplementary data
To read the full content of this article, please download the PDF.
Phasic respiratory mobility of both renal arteries with a larger deformation of the left renal artery stent at its mid segment, consistent with stent fracture.
Disappearance of phasic angulation of the left renal artery.

