
Abstract
Aims: The aim of this study was to assess the impact of drug-eluting stents (DES) compared to bare metal stents (BMS) for the endovascular treatment of atherosclerotic renal artery stenosis (ARAS).
Methods and results: We retrospectively evaluated all of our endovascular BMS and DES implantations performed in de novo ARAS between 2000 and 2014 at our institution. The occurrence of in-stent restenosis (ISR) detected by ultrasound or angiography, kidney function, blood pressure (BP), and the number of antihypertensive drugs were analysed as endpoints. Overall, 338 renal arteries were treated in 298 patients. BMS were implanted in 163 (48%), and DES in 175 lesions (52%). Of the 175 lesions treated with DES, 55 (31%) were treated with a BMS-in-DES hybrid technique. For reasons of comparability, only lesions treated with balloon sizes of 4-6.5 mm were included in the final analysis. After 12 months, the rate of ISR >50% was 18.6% in the BMS group and 7.2% in the DES group (p=0.031). None of the BMS-in-DES-treated (hybrid) lesions developed ISR (hybrid technique vs. BMS only p=0.008, hybrid technique vs. DES only p=0.034). Systolic BP and number of antihypertensive drugs remained unchanged in the BMS group but declined in the DES group (p=0.02). Renal function significantly deteriorated in the BMS group (p=0.03) but did not change significantly in the DES group (p=0.188).
Conclusions: DES were superior to BMS in preventing ISR. Overall, the BMS-in-DES-technique (hybrid) achieved the lowest risk for ISR.
Abbreviations
ARAS: atherosclerotic renal artery stenosis
BMS: bare metal stent
BP: blood pressure
DBP: diastolic blood pressure
DES: drug-eluting stent
GFR: glomerular filtration rate
ISR: in-stent restenosis
RAS: renal artery stenting
SBP: systolic blood pressure
Introduction
Atherosclerotic renal artery stenosis (ARAS) is a common cause of both secondary hypertension and chronic renal failure, reaching a prevalence of up to 7% in persons older than 65 years of age1. Despite encouraging early uncontrolled studies reporting a reduction in blood pressure (BP) and stabilisation of renal function, the published randomised trials have failed to demonstrate a benefit of renal artery stenting (RAS) using bare metal stents (BMS) only with concomitant medical therapy compared with best medical therapy alone2-5. However, all these trials focused on clinical outcome, not accounting for in-stent restenosis (ISR). Since ISR partly occurs in >30% of renal arteries following BMS implantation, this may be of clinical importance6,7. In the coronary vasculature, drug-eluting stents (DES) dramatically reduced ISR from nearly 30% in BMS-treated lesions to less than 10%8. However, data addressing renal ISR after DES implantation in de novo ARAS are limited9,10. Moreover, the question arises whether a reduction in ISR by using DES translates to better outcome in terms of renal function or BP levels.
Methods
PATIENT POPULATION
We retrospectively evaluated all patients who underwent endovascular stenting of de novo ARAS at both sites of our institution between December 2000 and June 2014 with respect to safety, comparative efficacy and long-term outcome. These patients were divided into two groups on the basis of the previously implanted stent type, the BMS group and the DES group. A subgroup, which comprised patients who were treated with a BMS-in-DES double-stenting technique, was classified as belonging to the DES group. Our criteria for revascularisation included unilateral or bilateral ARAS of more than 60% with concomitant severe hypertension while receiving two or more antihypertensive drugs and/or chronic kidney disease, which was defined as an estimated glomerular filtration rate (GFR) of less than 60 ml/min/1.73 m2, as calculated with the use of the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) formula11. The local ethics committee approved the study.
ENDOVASCULAR PROCEDURE
From 2000 to 2004 only BMS were used. The first renal DES implantation at our institution was performed in July 2004, since DES were first available in 2002 with diameters up to 5 mm. In a subset of patients, a stent-in-stent hybrid technique with implantation of a DES first followed by BMS implantation within the prior implanted DES was applied. This technique was mostly used to enhance the radial force of the DES with a BMS in the presence of recoil after plain DES implantation. Stent type and technique of implantation (BMS, DES or BMS-in-DES) as well as predilatation and post-dilatation were left to the discretion of the individual interventionalist. The decision was made on the basis of personal preference and experience, time of implantation, vessel size, presence of recoil after DES implantation and, amongst other things, feasibility of a longer-term dual antiplatelet therapy.
FOLLOW-UP
Clinical and duplex ultrasound follow-up visits were routinely scheduled three, six and 12 months after the index procedure and then annually. ISR was defined as a systolic peak flow velocity ratio >3.5, a systolic peak flow velocity >200 cm/sec measured inside or at the distal edge of the stent and/or a side-to-side difference (delta RI) of the intrarenal RI ≥0.05, as previously described12. Renal angiography was performed in case of suspected relevant restenosis or in the course of a scheduled cardiac catheterisation. Angiographic ISR was defined as ≥50% diameter stenosis as assessed by a core laboratory.
STATISTICAL ANALYSIS
Symmetrically distributed quantitative data are described by means and standard deviations, and the distributions of skewed data are summarised by median (minimum-maximum). For qualitative data, absolute and relative frequencies are presented. For comparability reasons the final statistical analysis was limited to patients treated with maximum balloon sizes of 4-6.5 mm, used for adjunctive post-dilatation, since no patient in the BMS group was treated with balloon sizes <4 mm and no patient in the DES group was treated with balloon sizes >6.5 mm.
Event time analysis methods were conducted for investigation of time from first procedure to restenosis. Cox regression models were fitted to the data to investigate the influence of quantitative parameters on the risk of restenosis and for the estimation of hazard ratios. A log-rank test stratified for maximum balloon size was applied in order to compare stent groups adjusted for balloon size. To test for within-group changes over time, t-tests for dependent samples were performed for quantitative data, and the sign test was used for discrete data (number of antihypertensive drugs used). For comparison of differences between groups, a two-sample t-test was used. The proportion of patients with increased, unchanged, and decreased number of antihypertensive drugs used was compared between groups with a chi-squared test. All tests were two-sided and a significance level of 5% was used.
Results
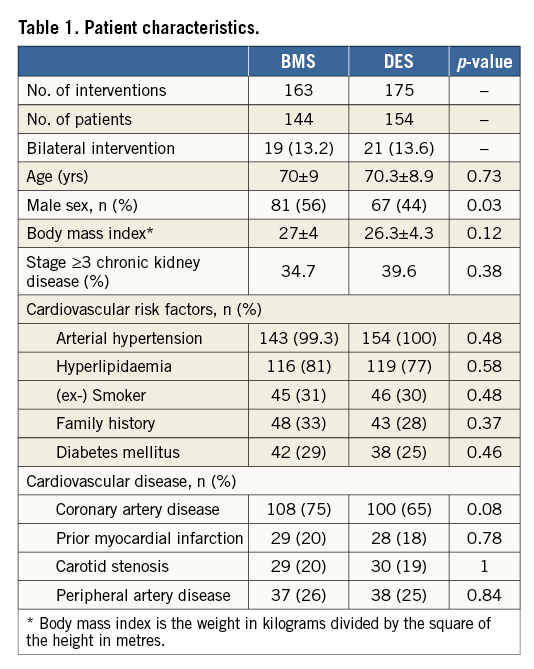
PATIENT CHARACTERISTICS
During the observation period, a total of 338 endovascular stent procedures were performed in 298 patients with de novo ARAS. BMS were implanted in 48% (n=163) and DES in 52% (n=175) of the lesions. In a subset of 31% (n=55) of patients with DES, lesions were treated with a stent-in-stent technique using both stent types. Bilateral interventions, either simultaneously or staged, were undertaken in 40 patients (13.4% of all patients). Apart from a significantly higher proportion of men in the BMS group, both groups were well balanced (Table 1).
LESION CHARACTERISTICS
Lesion characteristics are shown in Table 2. According to examiner-blinded QCA measurements, based on the angiographic data, the mean percent diameter stenosis of the renal artery prior to intervention was approximately 75% in both groups, and about 90% of the lesions were located at the ostium. The mean vessel diameter was significantly larger in the BMS as compared to the DES group, whereas the mean lesion length showed no significant difference. Furthermore, both stent length and diameter were significantly different between groups, with a larger stent diameter in the BMS group and a longer stent length in the DES group.
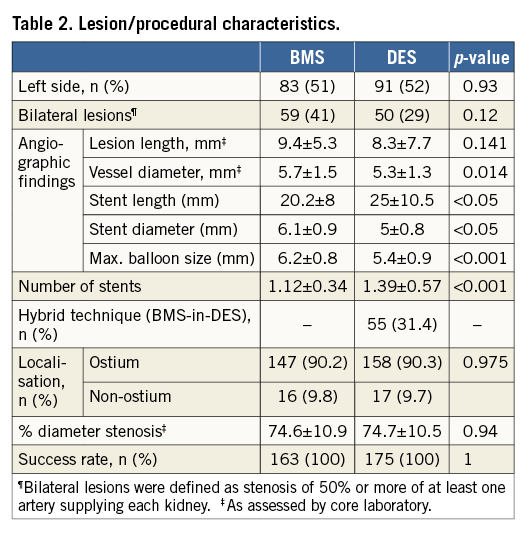
PROCEDURAL CHARACTERISTICS
The technical success rate, which was defined as successful stent deployment with a residual stenosis <30% at the end of the procedure, was 100% in both groups (Table 2). The following stent types were used in the BMS group: Herculink Elite® (Abbott Vascular, Santa Clara, CA, USA) (80%), MULTI-LINK ULTRA™ (Abbott Vascular) (8%), PALMAZ BLUE® (Cordis, Cardinal Health, Milpitas, CA, USA) (7%), MULTI-LINK RX PENTA (Abbott Vascular) (1%), Integrity™ BMS (Medtronic, Dublin, Ireland) (1%), Dynamic Renal (Biotronik, Bülach, Switzerland ) (1%), JOSTENT® (Abbott Vascular) (1%) and Driver® (Medtronic) (1%). The following stent types were used in the DES group: paclitaxel-eluting stents (TAXUS™; Boston Scientific, Marlborough, MA, USA) (87%), sirolimus-eluting stents (CYPHER®; Cordis) (6%), everolimus-eluting stents (XIENCE V®; Abbott Vascular) (4%), biolimus-eluting stents (Nobori®; Terumo Corp., Tokyo, Japan) (2%) and zotarolimus-eluting stents (Endeavor™; Medtronic) (1%). The hybrid BMS-in-DES technique was exclusively performed using the Herculink Elite BMS and the TAXUS DES with the aim of increasing radial strength in case of early recoil after DES implantation. On average, 1.39±0.6 and 1.1±0.3 stents were implanted per lesion in the DES and BMS groups, respectively (p<0.001).
The overall periprocedural complication rate was 4.4%, including six (1.8%) access-site complications comprising three false aneurysms, one haemodynamically insignificant AV fistula and two major bleedings, with one retroperitoneal bleeding requiring blood transfusion and one access-site bleeding with haemoglobin drop >2 mg/dl not requiring transfusion. Non-access-site associated minor bleedings with a haemoglobin drop <2 mg/dl occurred in two (0.6%) patients, comprising one macrohaematuria and one epistaxis. Further complications (0.9%) included two minor retrograde aortic dissections and one renal artery dissection requiring additional stenting. Four patients (1.1%) developed a transient contrast-induced nephropathy (CIN) with a post-procedural rise of the initial creatinine level by more than 25% compared to the base level, none of whom required dialysis.
Follow-up
PROCEDURAL OUTCOME
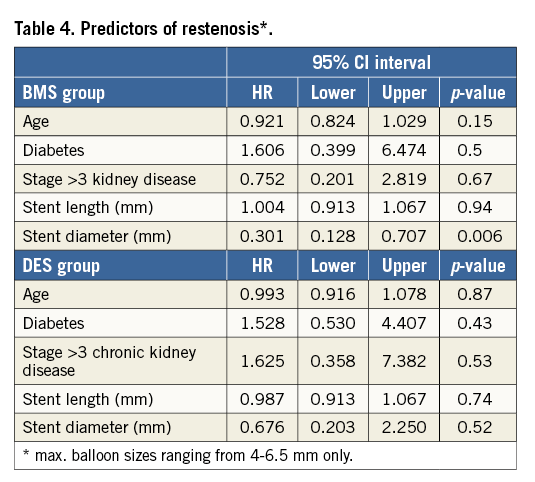
Follow-up data were available for 81% of all patients with a median follow-up time of 12 months (Table 3). Regarding our heterogeneous whole patient population, the rate of restenosis was 12% in the BMS group and 6.5% in the DES group (p=0.212) (Table 3). In the above-mentioned, quite comparable subset of patients with maximum balloon sizes ranging from 4-6.5 mm, the rate of ISR was 18.6% in the BMS group, but only 7.2% in the DES group (p=0.031) (Figure 1A). Moreover, the difference in favour of the DES group also persisted when different balloon sizes were compared (p=0.003) (Figure 1B). Of all investigated parameters, the only significant predictor of ISR was stent diameter, and this association was limited to the BMS group (Table 4).
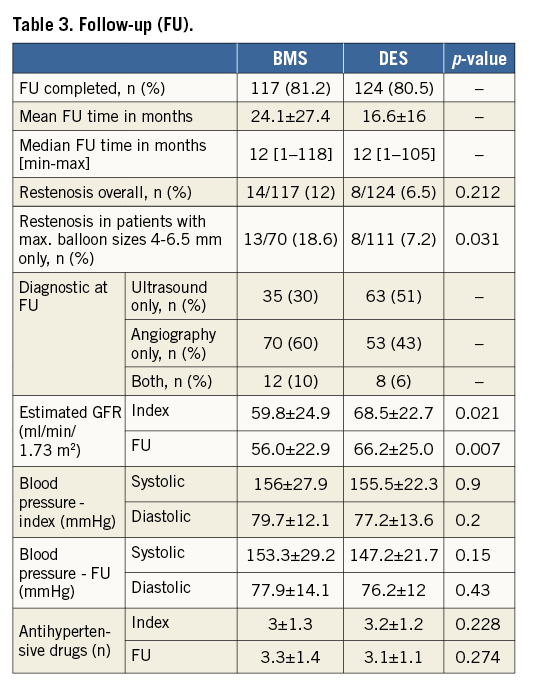
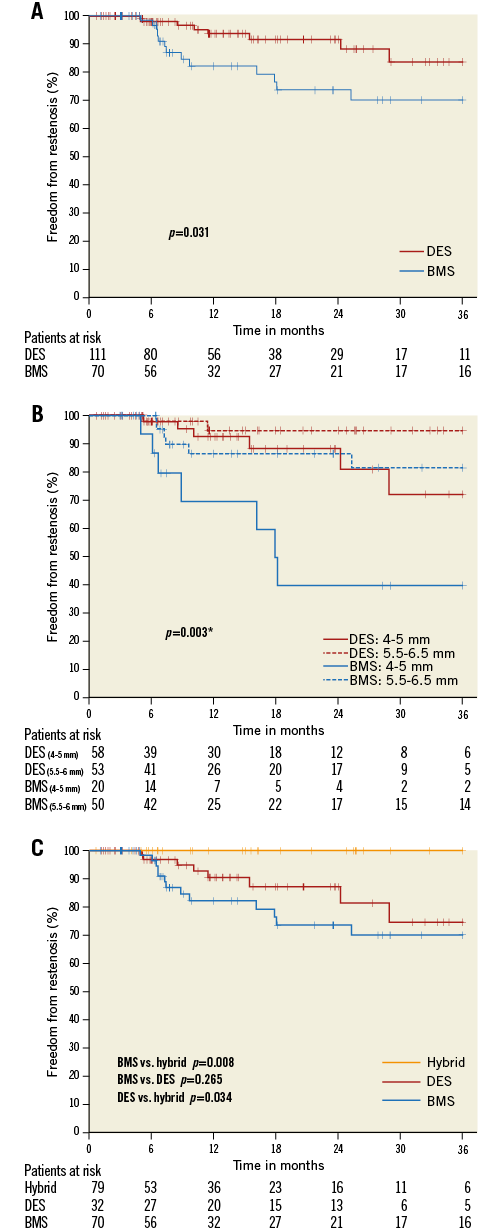
Figure 1. Three-year freedom from restenosis. Kaplan-Meier survival curves according to the occurrence of restenosis. A) Overall freedom from restenosis in BMS vs. DES. B) Freedom from restenosis depending on maximum balloon size in BMS vs. DES. * p-value stratified for maximum balloon size. C) Freedom from restenosis in BMS vs. Hybrid, BMS vs. DES and DES vs. Hybrid. BMS: bare metal stent; DES: drug-eluting stent; Hybrid: combination of DES/BMS
None of the lesions treated by the BMS-in-DES hybrid technique developed ISR. This group showed a significant benefit regarding the development of restenosis as compared to the BMS-only group (p=0.008), despite using the same BMS type (Herculink Elite) with comparable post-dilatation balloon sizes (BMS-only: 5.71±0.6 vs. BMS-in-DES: 5.77±0.5 mm, p=0.55) (Figure 1C). A similar benefit was also evident as compared to DES-only implantation (p=0.034) (Figure 1C). Although we observed a lower ISR with DES-only implantation compared to the BMS-only group, this difference was not statistically significant (p=0.265) (Figure 1C).
CLINICAL OUTCOME
When BP medication before stenting and after a median follow-up of 12 months was analysed, DES treatment was associated with a significant reduction of the number of drugs and fewer patients requiring an increase of the number of BP medications as compared to the BMS group (p=0.003) (Figure 2).
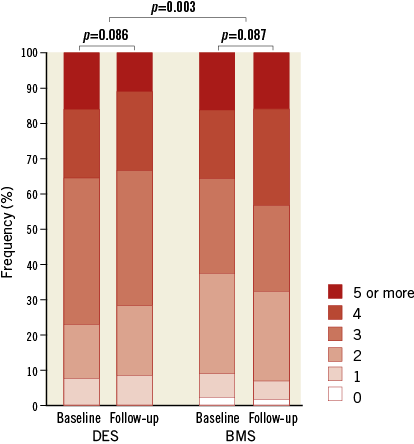
Figure 2. Number of antihypertensive drugs. Quantitative analysis regarding the number of antihypertensive drugs in patients with DES vs. BMS at baseline and at follow-up. BMS: bare metal stent; DES: drug-eluting stent
Systolic blood pressure (SBP) over time showed a significant reduction from 155.5±22.3 to 147.2±21.7 mmHg in the DES group (p=0.015) but a smaller and not statistically significant difference in the BMS group (156±27.9 vs. 153±29.2 mmHg; p=0.45) (Figure 3). Mean diastolic blood pressure (DBP) did not change relevantly in both groups (BMS group 79.7±12.1 vs. 77.9±14.1 mmHg; p=0.39; DES group 77.2±13.6 vs. 76.2±12 mmHg; p=0.54). Kidney function over time as assessed by GFR prior to the procedure and at follow-up demonstrated a significant decrease in the BMS group (59.8±24.9 vs. 56±22.9 ml/min/1.73 m2; p=0.03), while a smaller reduction was observed in the DES group (68.5±22.7 vs. 66.2±25 ml/min/1.73 m2; p=0.188) (Figure 4).
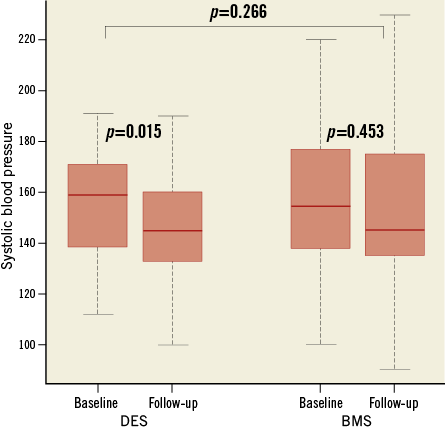
Figure 3. Systolic blood pressure levels. Box plot diagram showing systolic blood pressure levels (Y-axis, mmHg) in patients with BMS and DES at baseline and at follow-up. Horizontal lines denote median values. BMS: bare metal stent; DES: drug-eluting stent
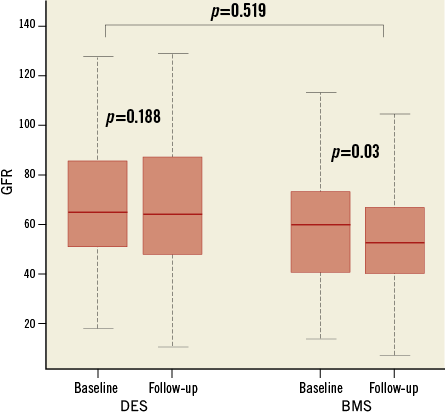
Figure 4. Kidney function. Box plot diagram showing kidney function as assessed by GFR (Y-axis, ml/min/1.73 m2) in patients with BMS and DES at baseline and at follow-up. Horizontal lines denote median values. BMS: bare metal stent; DES: drug-eluting stent
Discussion
The major findings of this retrospective cohort study of endovascular treatment of ARAS are that DES were associated with a lower incidence of ISR as compared to BMS, and that the lowest frequency of ISR was observed with a hybrid BMS-in-DES technique.
ENDOVASCULAR THERAPY OF ARAS
Based on promising clinical evidence derived from uncontrolled studies in the late 1990s and early after 2000, randomised clinical trials comparing stent revascularisation of ARAS in addition to optimal medical therapy or to medical treatment alone have so far failed to demonstrate significant advantages in BP lowering, preservation of renal function or reduction of cardiovascular morbidity and death2-4. However, none of these trials evaluated procedural outcomes such as the development of ISR, and mechanistic insight into the null treatment effect was lacking. Moreover, the published rate of ISR after BMS implantation within the renal vasculature varies between 3.5% and ~20%, and in patients with a renal vessel diameter <4.5 mm an ISR rate up to 36% has been reported6,7,13,14. Therefore, the development of ISR after BMS implantation may potentially have a substantial impact on clinical outcome within the intervention groups of the large comparative trials which all exclusively implanted BMS.
DES FOR ENDOVASCULAR THERAPY OF ARAS
It should be noted that DES implantation in patients with ARAS is off-label and that there are currently no dedicated renal DES commercially available. However, DES implantation previously demonstrated incremental benefits in preventing restenosis in coronary arteries8, and recently DES implantation also emerged as an effective treatment option in non-coronary arteries of comparable vessel size such as the lower limb15. However, there is sparse evidence for the usage of DES in renovascular disease. Currently, only two clinical trials, both not randomised, comparing DES with BMS in patients with de novo ARAS9,10 have been published. The GREAT trial showed a reduction of ISR from 14.6% to 6.7% in patients receiving DES in comparison to BMS. However, due to the small patient number of around 50 in each group, the difference was not statistically significant, although the rate of ISR in the DES arm was numerically only half that observed in the BMS group9. Furthermore, in a much smaller study including only 27 ARAS patients, a non-significant trend towards a higher restenosis-free patency rate after two years of 68% for the use of DES and 47% for BMS was found, despite a significantly smaller lesion diameter of 3.4±0.6 mm in the DES group as compared to 5.3±0.6 mm in the BMS group10.
The overall rate of restenosis in our study regarding all BMS- and DES-treated patients showed no significant difference despite a reduction of restenosis from 12% (BMS group) to 6.5% (DES group). This is possibly due to a selection bias with significantly smaller stent, balloon, and vessel diameters as well as significantly longer stents in the DES group, all known predictors of restenosis at least in coronary artery disease8.
The ISR rate in our quite comparable subset of patients with maximum balloon sizes of 4-6.5 mm was 7.2% in the DES group, which was significantly lower than that in the BMS group, where it averaged 18.6%. While stent diameter was the major predictor for development of ISR in BMS, smaller vessels treated by DES demonstrated no significant increase in ISR.
Several factors may contribute to the development of ISR after stenting of ARAS. Excessive neointimal proliferation or mechanical recoil, particularly in ostial lesions due to calcification or rigid aortic plaques, as well as inadequate stent implantation without covering of the ostium, may contribute to the reappearance of renal artery stenosis16,17. In our study, DES-only implantation showed a reduction of ISR in comparison to BMS but still 9.3% of these patients developed restenosis at follow-up, which was a non-significant difference. This was partly caused by neointimal proliferation but may also reflect recoil due to stent compression. The most commonly used DES in our study was the TAXUS stent since it was the only DES which was available in diameters up to 5 mm. However, this stent type, which was originally developed for the coronary system, exhibits only a limited radial strength, thus facilitating stent compression in cases of heavily rigid plaque composition, which is likely to occur in the renal vasculature. Another potential reason for lack of significance in restenosis when comparing DES-only patients to the BMS group might be stent overexpansion with larger balloons, which probably diminishes the radial force of the DES even more and might also reduce the effective drug dose applied to the vessel wall.
The Herculink Elite BMS which was developed to prevent recoil offers a much higher radial strength as compared to the TAXUS DES, which is particularly needful in ostial lesions which account for approximately 90% of the lesions treated in our cohort and which also represents the majority of ARAS location in the literature18. Therefore, in the case of a larger vessel diameter or in case of early recoil, we usually perform a hybrid technique in which an additional Herculink Elite BMS is deployed within a DES. This hybrid technique (also called double-stenting technique) has also been described for the treatment of coronary lesions with acute stent recoil or luminal filling defects and showed improved angiographic outcome with good clinical results in the medium term19. Notably, none of the lesions treated with this double-stenting technique in our cohort developed ISR, thus suggesting that the application of drug elution to prevent neointimal proliferation combined with enhanced radial stent force to prevent recoil probably represents the optimal endovascular approach for ARAS treatment.
FEASIBILITY OF ARAS STENTING
RAS at our high-volume centre was feasible and safe. The technical success rate was 100%, which compares well with published data from other trials ranging from 95-100%2-4,9. The complication rate in experienced hands at our institution was very low and most commonly included access-site complications. Complication rates of up to 20% including substantial risks and even death reported in other studies may be related to operator experience or low-volume institutional numbers, thus emphasising the importance of well-trained interventionalists and the appropriate hospital setting3,4.
CLINICAL OUTCOME
In our study, BP control and renal function were favourable in the DES-treated group. Patient characteristics were equally distributed between groups; however, it may only be speculated that the reduction of ISR by DES may be causally related to the significantly better reduction of systolic BP, number of antihypertensive drugs as well as to the preservation of renal function over time. Regarding renal function, it should be noted that patients in the BMS group started with a significantly lower GFR at baseline, and further decrease of renal function may not necessarily be caused by a reduced perfusion due to restenosis but may also be a result of other underlying pathological processes which may not have been detected in this retrospective analysis.
Since no randomised trials are available to date comparing DES and BMS implantation in patients with ARAS, our data currently represent the largest comparative efficacy study and demonstrate for the first time that revascularisation of ARAS using DES and/or the application of a dedicated hybrid stenting technique is superior to BMS with respect to the occurrence of ISR. Further randomised studies are warranted to provide stronger evidence in relation to comparative efficacy as well as to evaluate its influence on clinical outcome parameters in patients with ARAS.
Limitations
This is a single-centre retrospective analysis with all the known limitations of such a study. Decision making before RAS was based on visual estimation of the stenosis and not on functional measurements. A selection bias regarding time of intervention as well as the type of stent (DES or BMS) or implantation technique (e.g., double-stenting) cannot be excluded. Follow-up was incomplete in one fifth of the patients. Moreover, surveillance was performed using different imaging modalities including angiography or ultrasound. Despite the fact that duplex ultrasound is a well-established and accurate technique to identify significant restenosis in stented renal arteries, an underestimation of ISR by the more frequent use of ultrasound as follow-up in the DES group cannot be entirely excluded. On the other hand, Chi et al found that current duplex criteria for native renal arteries may rather overestimate the degree of ISR because, due to changes in vessel compliance, peak systolic velocity for instance is likely to be higher for any given degree of arterial narrowing within the stent20. It remains unclear whether the double-stenting technique may have altered the ability to measure the in-stent velocity, possibly leading to false-negative results. However, 60% of follow-up within this cohort was performed by angiography, which is comparable to that of the BMS group. Finally, results concerning the clinical outcome should be considered with caution due to the non-randomised, retrospective nature of this study, and BP levels were mostly derived from multiple office-based measurements with all their known limitations.
Conclusions
In a cohort of almost 300 patients with renal artery stenosis, the use of DES was associated with a lower ISR compared to BMS. Overall, the lowest ISR was observed in lesions treated with a double-stenting BMS-in-DES technique that provides both an antiproliferative benefit and increased radial force. Furthermore, DES were associated with a lower systolic BP and fewer antihypertensive drugs as compared to BMS.
| Impact on daily practice This study demonstrates a significant reduction of ISR with DES in comparison to BMS in patients with ARAS. Overall, the lowest rate of ISR was observed in lesions treated with a double-stenting BMS-in-DES technique. Therefore, whenever renal artery stenting is indicated, according to current guidelines, clinicians should use DES wherever possible. The BMS-in-DES hybrid technique, which provides both an antiproliferative benefit and increased radial force, should be applied in the case of early recoil or in ostial lesions with high plaque burden. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

