Abstract
Aims: Microvascular obstruction (MVO) and “no-reflow phenomenon” (NRP) remain barriers to optimal tissue perfusion after percutaneous coronary intervention (PCI). The purpose of this study was to develop, characterise, and test an adenosine-eluting guidewire (Adenowire) for coronary vasodilation.
Methods and results: Utilising polyurethane chemistry, we developed a non-toxic pentameric form of adenosine (PA) that can be coated onto guidewires (Adenowire) and that allows continuous elution of adenosine into the distal vascular bed during PCI. We characterised PA with Fourier transform infrared spectroscopy, NMR and MALDI time-of-flight mass spectrometry, established its stability by calorimetry, and confirmed its safety by extensive toxicological testing. Adenowires reliably released adenosine in vitro over 60 minutes. In pigs, insertion of an Adenowire into the left circumflex or left anterior descending coronary artery resulted in immediate and sustained (40 minutes) vasodilation. Electron microscopy demonstrated smooth thin coating of the terminal portion of guidewires and showed lack of fibrin or platelet adhesion to the Adenowire after in vivo use.
Conclusions: Since guidewires are the first devices to cross a culprit lesion, Adenowires would prophylactically medicate vascular beds with adenosine at the target site without the need for additional manipulations by the interventionalist.
Introduction
Percutaneous coronary intervention (PCI) is the most frequently performed modality to treat atheromatous-induced stenosis of coronary arteries and degenerative saphenous vein grafts in numerous clinical scenarios, including ST-segment elevation myocardial infarction (STEMI)1. While PCI invariably restores epicardial vessel patency, the deployment of a variety of interventional equipment in the coronary vasculature may result in impairment of tissue perfusion through microvascular obstruction (MVO) which manifests angiographically as the “no-reflow” phenomenon (NRP)2-5. The incidence of MVO varies from approximately 5% in stable patients to more than 50% in STEMI patients undergoing PCI, and evidence suggests that MVO is an independent predictor of early and late mortality5-10. The mechanisms of MVO include mechanical obstruction of capillaries via cellular elements, atheromatous debris and microthrombi and intense vasoconstriction of the microvasculature2,3,5,11.
There is a need for new approaches to prevent and reverse microvascular dysfunction associated with PCI2,5,6. Adenosine, an endogenous nucleoside, functions as a local hormone and activates four receptors producing a myriad of physiological effects which would be expected to maintain microcirculatory flow during PCI, with reversal of vasoconstriction and antiplatelet activity being paramount3,4,12. Intravenous adenosine infusions in animals and humans result in myocardial salvage partly due to preservation of microcirculatory flow4,13,14. Also, adenosine evokes numerous biochemical pathways that reduce myocyte necrosis4. Although most studies demonstrate beneficial effects of adenosine in reducing MVO, it is likely that its full therapeutic potential is compromised due to adenosine’s extremely short half-life in blood (approximately one second) and because of dilution of the administered adenosine when delivered via a guiding catheter or balloon catheter. We hypothesise that prophylactic and continuous delivery of adenosine selectively in the intervened vascular bed during a procedure would optimise adenosine’s cardioprotective effects. Utilising polyurethane technology, we developed a unique, non-toxic adenosine pentamer (PA) in which five adenosine molecules are covalently bonded via four methyllysine molecules. PA can be coated on guidewires, and upon contact with body fluids PA hydrolyses to release free adenosine. We call PA-coated guidewires “Adenowires”. This report describes the synthesis and characterisation of PA, as well as the elution characteristics of adenosine from Adenowires. We also present preliminary safety and efficacy data for Adenowires in a porcine model.
Methods
SYNTHESIS OF PA
Lysine diisocyanate methyl ester (LDI) was purchased from Kyowa Hakko Kogyo (Tokyo, Japan) and purified by vacuum distillation. Other reagents were from Sigma-Aldrich (Milwaukee, WI, USA). PA was synthesised in two steps using a method adapted from Zhang and co-workers15,16. In step one (Figure 1), adenosine (1 mmole), 2 ml of dimethyl sulfoxide (DMSO) and LDI (4 mmoles) were placed in a flask and sealed under nitrogen. The reaction mixture was stirred in the dark at room temperature for two days. In step one, the functional groups (hydroxyl and amine groups) of adenosine were capped with LDI to produce the isocyanate-terminated monomer (Adenosine-LDI monomer) shown in Figure 1. The reaction between adenosine and LDI accompanying formation of urethane linkages was monitored by Fourier transform infrared spectroscopy (FT-IR).

Figure 1. Schematic illustrates the first step in the synthesis of pentameric adenosine. Lysine diisocyanate methyl ester (LDI) covalently bonds to the amino and hydroxyl groups of adenosine to produce the intermediate, Adenosine-LDI monomer.
The second reaction step (Figure 2) was initiated by the addition of adenosine (4 mmoles in 2 ml of DMSO) to the reaction mixture when FT-IR indicated that 50% of isocyanate groups (peak at 2,279 cm–1) initially present had been consumed (Figure 3). The reaction mixture was then stirred at room temperature for another two days until FT-IR showed no remaining isocyanate groups. The likely structure of PA yielded by this reaction is shown in Figure 2.

Figure 2. Schematic illustrates the second step in the synthesis of pentameric adenosine. Amino groups are more reactive to isocyanates than are hydroxyl groups, so most adenosine molecules probably covalently bond to the Adenosine-LDI monomer via their amine groups (although some linkages probably also involve hydroxyl groups).
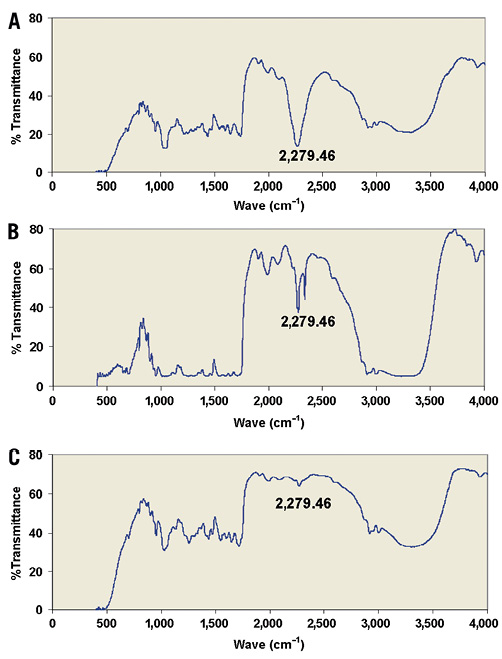
Figure 3. The isocyanate groups in lysine diisocyanate methyl ester (LDI) give a strong Fourier transform infrared spectroscopy (FT-IR) signal at 2,279 cm–1 (A). Consumption of half the isocyanate groups by the first reaction (Figure 1) with adenosine results in a 50% reduction in the 2,279 cm–1 signal (B), and consumption of the remaining isocyanate groups by the second reaction (Figure 2) with adenosine results in complete loss of the FT-IR signal at 2,279 cm–1 (C).
CHARACTERISATION OF PA
To characterise PA, 1H NMR spectra were obtained for adenosine, LDI and PA in DMSO with a Varian INOVA 600 MHz instrument (Varian, Inc., Palo Alto, CA, USA). Also, a mass spectrum of PA was obtained with a Voyager STR high performance MALDI time-of-flight (TOF) mass spectrometer (Applied Biosystems, Foster City, CA, USA). Glass transition temperature (Tg) was measured with ellipsometry using a Woollam M-2000 ellipsometer as previously described17.
SCANNING ELECTRON MICROSCOPY
Scanning electron microscopy (SEM) was performed on Kinetix® wires (Boston Scientific, Natick, MA, USA) under four different experimental conditions (control wires with no coating, wires coated with one to three applications of PA utilising a dipping method, and coated and control wires inserted into porcine coronary arteries for 40 minutes) with a Topcon DS130F field emission scanning electron microscope (Topcon, Livermore, CA, USA). Parameters analysed were thickness and smoothness (in vitro) and amount of residual PA and adhesion of platelets, leukocytes and fibrin on wires (in vivo).
GUIDEWIRE COATING
Cougar® (Medtronic, Minneapolis, MN, USA) and Kinetix® (Boston Scientific Corp.) 0.014” coronary guidewires were utilised for coating with PA. The distal 28 cm portion of the wire was immersed in DMSO for two hours, rinsed with deionised water for five minutes and allowed to dry at room temperature for 24 hours. Further drying was accomplished with a Savant Speedvac (Thermo Electron Corporation, Waltham, MA, USA) for one to two hours. PA in DMSO (2 ml) was placed in a sealed glass tube protected from light to which 200 mg of free adenosine was added. Thus, the final coating material utilised to coat the guidewires consisted of 2.185 grams (1 mmole) of PA and 0.200 grams of adenosine in 4 ml of DMSO. A pipette tip was dipped into the suspension and then slowly moved across a horizontally suspended wire and repeated until 28 cm of the wire was evenly coated. The wire was allowed to dry at room temperature for 12 hours and then the coating process was repeated two times. Following application of the third coat the wires were dried for 48 hours at room temperature and then in a Speedvac for six hours. For every 10 cm of wire length, the final coated wire contained 0.33 µmoles (0.72 mg) of PA and 2.67 µmoles (0.71 mg) of free adenosine (1.15 mg of total adenosine, i.e., free adenosine + adenosine capable of being released from the PA). For a 20 cm coating, these values would be doubled.
CYTOTOXICITY TESTING
Cytotoxicity testing was performed by NAMSA (Northwood, OH, USA) based on the International Organization for Standardization 10993-5. A guidewire coated as described above was eluted with Minimum Essential Medium supplemented with 5% foetal bovine serum, antibiotics (100 units/mL penicillin, 100 μg/mL streptomycin and 2.5 μg/mL amphotericin B) and L-glutamine (2 mM).Triplicate monolayers of mouse fibroblast cells were treated with this test article and incubated at 37°C in the presence of CO2 for 48 hours and examined microscopically for abnormal cell morphology and degeneration.
HAEMOLYSIS TESTING
Haemolysis testing was performed by NAMSA based on the International Organization for Standardization 10993-4. Stainless steel plates (35 to 44 cm2) were coated with the same product used to coat the guidewires (this provided more surface area than practical with coated guidewires). The product was then eluted from the steel plates with calcium and magnesium free phosphate buffered saline (12 to 15 ml). Anticoagulated whole rabbit blood was pooled, diluted and added to tubes with these test articles in calcium and magnesium free phosphate buffered saline. Following incubation for three hours at 37°C, the tubes were centrifuged and supernatant collected and analysed using a spectrophotometer at 540 nm wavelength.
ACUTE SYSTEMIC TOXICITY TESTING
Acute systemic toxicity testing was performed by NAMSA based on the International Organization for Standardization 10993-11. Stainless steel plates (38-40 cm2) were coated with the same product used to coat the guidewires. The product was then eluted from the steel plates with either 0.9% saline (13 ml) or sesame seed oil (13 ml). These test articles were injected either intravenously (saline extract) or into the peritoneal cavity (sesame seed oil extract) into five mice for each group. Animals were observed for signs of toxicity immediately after injection and at 4, 24, 48, and 72 hours after injection.
PYROGENICITY TESTING
Pyrogenicity testing was performed by NAMSA based on the International Organization for Standardization 10993-10. Stainless steel plates (total area=477 cm2) were coated with the same product used to coat the guidewires. The product was then eluted from the steel plates with 0.9% saline (159 ml), and a single dose of 10 ml/kg was injected IV into the marginal vein of three rabbits. Rectal temperatures were recorded prior to and at 30-minute intervals for three hours after injection.
SENSITISATION AND IRRITATION TESTING
Sensitisation and irritation testing was performed by NAMSA based on the International Organization for Standardization 10993-10. Stainless steel plates (37 to 45 cm2) were coated with the same product used to coat the guidewires. The product was then eluted from the steel plates with either 0.9% saline (15 ml) or sesame seed oil (12 ml). These test articles were injected intradermally and occlusively patched to 15 guinea pigs (10 test and 5 control). Following a recovery period, the animals received a challenge patch of test article or control, and sites were scored for dermal reactions at 24 and 48 hours after patch removal. In irritation studies the test articles were injected subcutaneously into the back of three rabbits at five separate sites and observed for erythema at 24 and 48 hours.
IN VITRO DRUG ELUTION
These studies were performed in 0.9% saline rather than in plasma or blood because adenosine has a short half-life (seconds) in the latter two media, making detection difficult. Tubes containing 15 ml saline were maintained at 37°C, and 11 cm of the distal coated end of the guidewire was placed serially in tubes for 0-5, 5-10, 10-20, 20-30, 30-40, 40-50, and 50-60 minutes. One-ml samples were taken from the tubes to measure the concentration of adenosine utilising high performance liquid chromatography with fluorescence detection as previously described18.
PORCINE STUDY
All porcine studies were approved by the Institutional Animal Care and Use Committee of St. Joseph Translational Research Institute. Yorkshire swine were premedicated with intramuscular ketamine (20 mg/kg), acepromazine (1.1 mg/kg) and atropine (0.05 mg/kg). Anaesthesia was maintained with 1% isoflurane and supplemental oxygen to maintain a pH at 7.4±0.06. Animals received aspirin (300 mg orally for three days) prior to the study and were anticoagulated throughout the procedure with heparin (150 units/kg). Heart rate and EKG changes were monitored continuously utilising leads I, AVF, and AVL. A 7 Fr sheath was placed in the femoral artery. The left or right coronary artery ostium was engaged with a 6 Fr HS guide catheter and coronary angiography performed to confirm vessel patency.
For the first set of experiments, coronary blood flow (CBF) was measured utilising a 0.014 inch pressure/temperature sensor-tipped guidewire (PressureWire 5; Radi Medical Systems, Uppsala, Sweden) connected to a dedicated interface (Radi Analyzer; Radi Medical Systems) with modified software for online analysis of thermodilution curves19,20. The wire was placed in the mid portion of the left anterior descending (LAD) or right coronary artery (RCA) and the baseline mean transit time (Tm) was obtained with injection of 3 ml of room temperature saline in triplicate into the guide catheter. CBF was then calculated from Tm which is inversely proportional to coronary flow. A Medtronic Cougar 0.014” guidewire (Medtronic, Minneapolis, MN, USA) which had been coated with PA on the distal 28 cm was positioned in the distal third of the vessel containing the Radi wire. Tm were obtained serially for 30 minutes and repeated five minutes after wire removal.
In the second set of experiments coronary blood flow velocity (CBFV) was measured utilising a 0.014” Doppler guidewire (Flowire®; Volcano Corporation, Rancho Cordova, CA, USA) and dedicated software. CBFV was measured as continuous pulsatile velocity (cm/sec) and mean values were used in calculations. The Doppler wire was positioned in the mid portion of the LAD or circumflex (LCX) vessels and baseline CBFV obtained. Care was taken to ensure the wire remained in the same position throughout the experiment. A bolus dose of adenosine (120 μg) was given through the guide catheter and peak CBFV reserve obtained. After CBFV had returned to baseline a Kinetix guidewire coated with PA was rapidly positioned in the distal third of the epicardial vessel containing the Doppler wire, and CBFV was obtained serially over the next 40 minutes. The wire was then removed and CBFV was repeated five minutes later. In some animals, an uncoated wire was positioned in an identical position and velocities measured over 20 minutes. After removal, the wires were immediately placed in 3% glutaraldehyde for electron microscopy studies.
STATISTICS
Statistical analysis was performed by repeated measures one-factor analysis of variance. The criterion of significance was p<0.05.
Results
SYNTHESIS OF PA
At the first step of synthesis, the FT-IR spectrum obtained from the reaction mixture exhibited peaks centred at 2,279 cm–1 associated with the isocyanate group (Figure 3A) which was not found in the FT-IR spectrum of adenosine. This peak was reduced by 50% in the FT-IR spectrum of the reaction mixture two days after initiating the reaction (Figure 3B) indicating that all functional groups of adenosine were bonded to an isocyanate group. The FT-IR spectrum demonstrated urethane linkages, as evidenced by the peak at wave numbers ranging from 1,610 to 1,750 cm–1. Two days into the second step of synthesis, there was quantitative consumption of isocyanate groups as noted by the complete disappearance of the FT-IR signal at approximately 2,279 cm–1 (Figure 3C). The peak maximum of the ester carbonyl was at 1,747 cm–1. The carbonyl peak of the urethane was at 1,658 cm–1 and that of the urea was at 1,631 cm–1. FT-IR spectrum therefore indicated that adenosine had bonded with LDI and PA had formed.
CHARACTERISATION OF PA
The 1H NMR spectra for adenosine, LDI and PA are shown in Figure 4 and were consistent with their presumed structure. The chemical shifts for adenosine protons were represented in the NMR for PA, indicating the presence of adenosine in the product. Notably, the chemical shifts for LDI protons were different in PA, indicating bond formation by LDI. MALDI-TOF demonstrated abundant fragments at 268, 352, 395, 451, 452, 581, 640 and 767 m/z (positive ion mode) that were consistent with the presumed structure of PA. The glass transition temperature (Tg) of PA was 119±1°C (Figure 5) indicating stability of PA even at high temperatures.
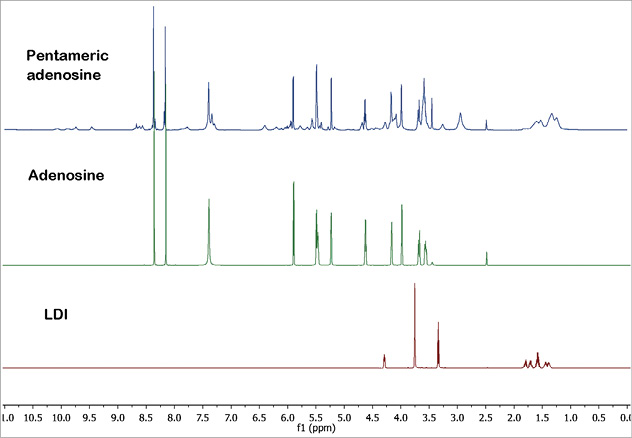
Figure 4. Proton NMR spectra of adenosine, lysine diisocyanate methyl ester (LDI) and pentameric adenosine in DMSO-D6 are shown.
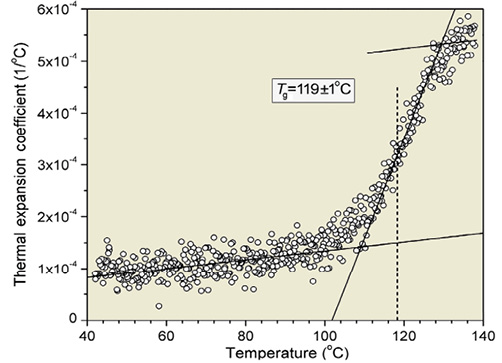
Figure 5. Ellipsometry was used to measure glass transition temperature (Tg), which was 119±1°C (identified as the mid-point of the change in thermal expansion coefficient from the liquid-to-glassy state).
SCANNING ELECTRON MICROSCOPY
Micrographs of the distal end of a guidewire coated with PA are shown in Figure 6. The coating provided a smooth surface with a thickness of four to six microns after three coats of PA. Residual PA was still adherent to the wires after 40 minutes insertion into the porcine coronary artery (Figure 7A and Figure 7B). There was no difference in the amount of fibrin or cellular deposition when compared with uncoated wires inserted in vivo (Figure 7C and Figure 7D).
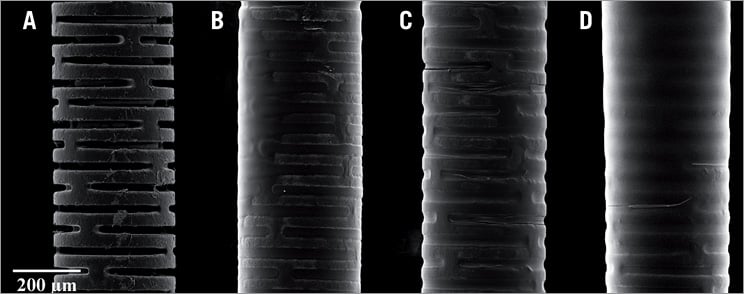
Figure 6. Scanning electron micrographs of distal end of coronary guidewires from manufacture (A) and after one (B), two (C), and three (D) coats of pentameric adenosine. Note the smooth surface with an average thickness of 4-6 microns.
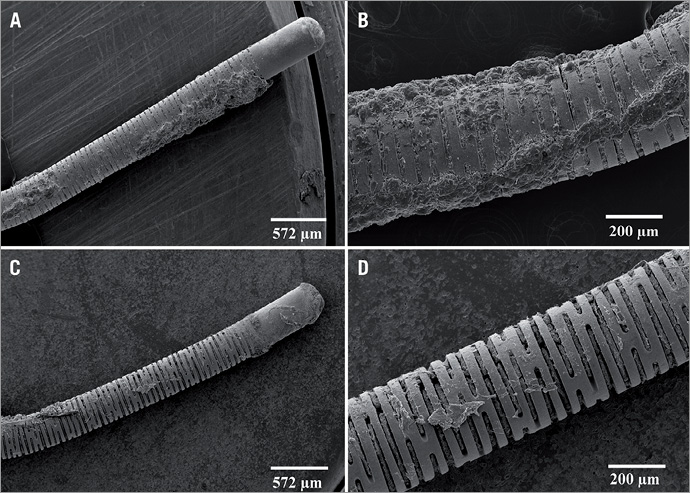
Figure 7. Scanning electron micrographs of the distal end of a 0.014” coronary guidewire following insertion in the porcine coronary artery. Note that residual pentameric adenosine was still adherent to the wires after 40 minutes (A & B). There was no difference in the amount of fibrin or cellular deposition when compared with uncoated wires inserted in vivo (C & D).
SAFETY TESTING
All tests confirmed that PA met standards for safety. An extract of PA-coated guidewires or stainless steel plates showed no evidence of causing cell lysis or toxicity. The mean haemolytic index for extracted PA was 0.0% and hence PA was considered non-haemolytic. There was no mortality or evidence of systemic toxicity from the extract. Pyrogen tests showed that PA was non-pyrogenic. PA showed no evidence of causing delayed contact dermal sensitisation or irritation.
IN VITRO DRUG ELUTION
Studies of cumulative elution of adenosine over 60 minutes showed an initial burst of drug release (0.6 mg) in the first five minutes which probably represents release of free adenosine in the coating (Figure 8). The subsequent release over the next 50 minutes was likely due to non-enzymatic hydrolysis of the covalent bonds linking adenosine to PA. Such a kinetic profile is desirable for an interventional procedure since the initial burst would treat and the sustained release prevent MVO. Since only approximately 11 cm of the 28 cm coated portion of the wire was exposed to saline, the potential cumulative release can be projected to be 2.5-3.0 mg of adenosine.
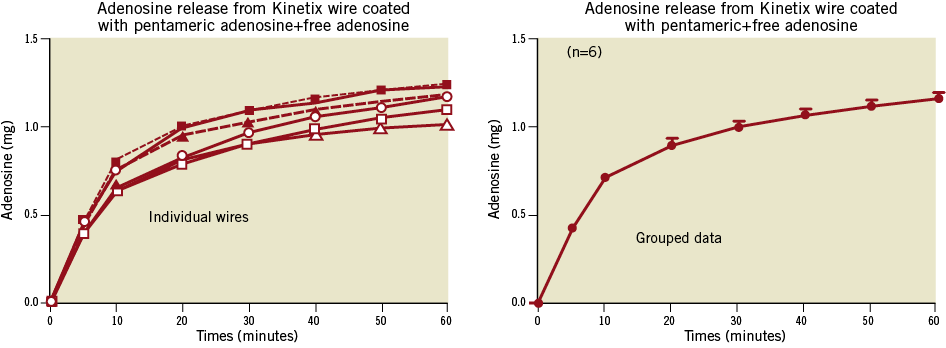
Figure 8. Illustration of the time course of adenosine release from six Adenowires in vitro (water bath at 37°C). Left panel shows the results for each individual wire and right panel shows means±SEM for the grouped data.
PORCINE STUDY
PA-coated Medtronic Cougar guidewires increased coronary blood flow fivefold in the first 10 minutes after insertion, and this response dissipated over the ensuing 20 minutes (Figure 9). Subsequent studies utilising a micro-machined guidewire (Figure 10) demonstrated a prolonged response, as would be expected from the in vitro kinetic studies. This vasodilatory response was more suitable for the duration of a typical interventional procedure. CBFV increased more than twofold in the first five minutes and remained significantly increased over the ensuing 35 minutes (Figure 10). The peak increase in CBFV with the Adenowire was similar to a 120 µg intracoronary bolus of adenosine through the guidewire catheter which resulted in a mean increase of 219±14%. However, compared with the Adenowire the vasodilatory effect only persisted for 90±15 seconds. No changes in heart rate, blood pressure or rate-pressure-product were observed during the PA-coated guidewire placement and no animals developed heart block. CBFV measured in three animals with a control wire remained unchanged. Coronary angiograms showed TIMI 3 flow throughout the experimental protocol. When the PA-coated guidewires were removed, CBFV returned to basal within five minutes, indicating pharmacological activity up to 40 minutes. PA-coated guidewire performance (torque ability and tip flexibility) was similar to uncoated wires from the same manufacturer (n=3).
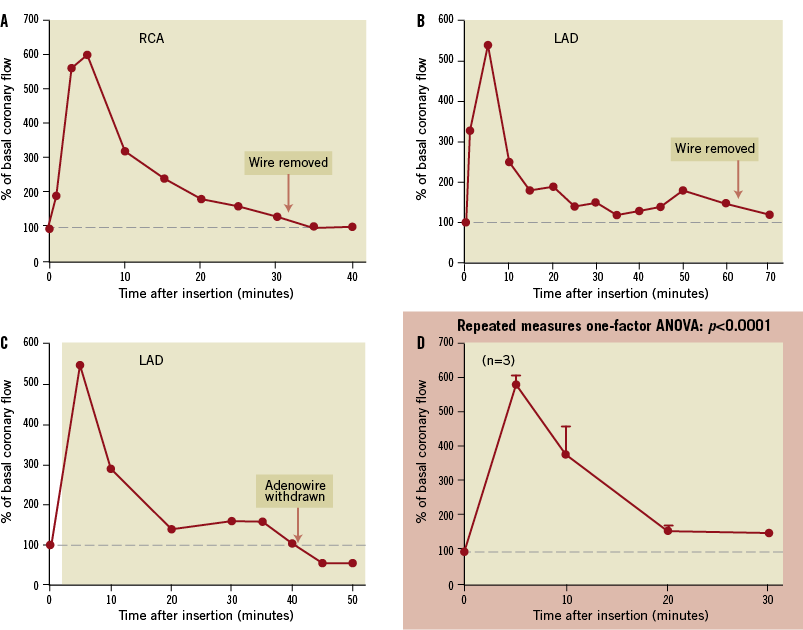
Figure 9. Medtronic Cougar® guidewires coated with pentameric adenosine were inserted into the right coronary artery (RCA; A) or left anterior descending coronary artery (LAD; B and C) of pigs. A marked increase (approximately fivefold) in coronary blood flow occurred within the first 10 minutes after insertion which rapidly dissipated over the ensuing 20 minutes. Group data (means±SEM) are shown in panel D.
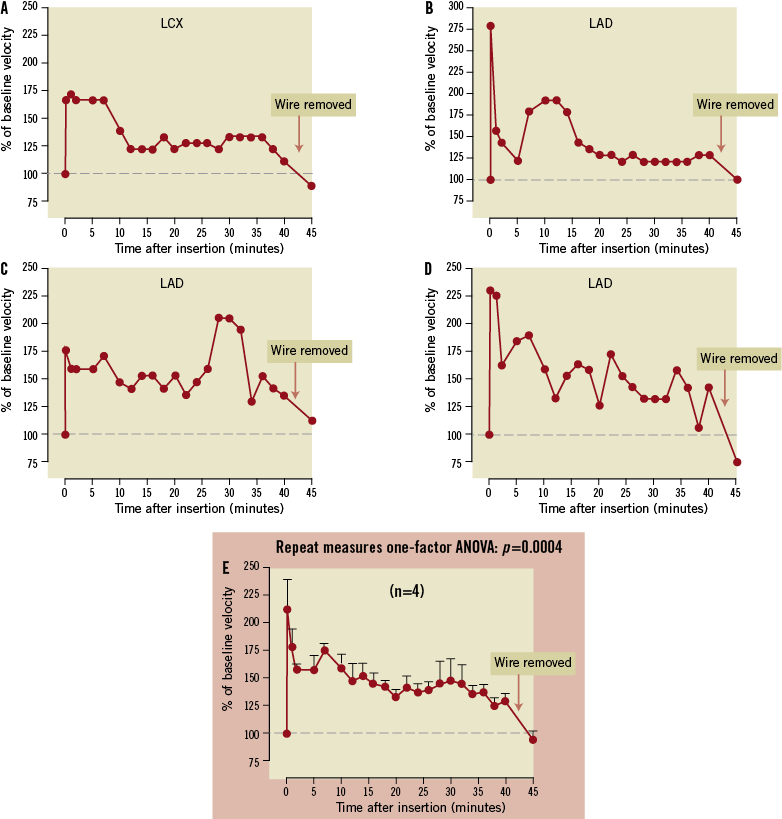
Figure 10. Kinetix® guidewires coated with pentameric adenosine were inserted into the left circumflex coronary artery (LCX; A) or left anterior descending coronary artery (LAD; B, C and D) of pigs. A marked increase (approximately twofold) in coronary blood flow occurred within minutes after insertion, and coronary blood flow was maintained significantly over basal for 40 minutes. Removal of the Adenowire resulted in return of coronary blood flow to basal levels. Group data (means±SEM) are shown in panel E.
Discussion
Studies show that LDI can be used to generate urethane linkages15,16, and we use this approach to prepare PA. NMR experiments corroborate PA’s structure and relative purity. Amino groups are more reactive to isocyanates than are hydroxyl groups, so most adenosine molecules probably covalently bond to the Adenosine-LDI monomer via their amine groups. Although some linkages probably also involve hydroxyl groups, this is a distinction without a difference, since PA would release adenosine regardless. Calorimetry studies show that PA is thermally stable, indicating that PA would be stable at ambient temperatures. In vitro experiments suggest that PA undergoes non-enzymatic hydrolysis when exposed to an aqueous solution, resulting in rapid release of adenosine over a time period suitable for interventional procedures. An array of tests shows that PA is safe. Porcine studies verify that pharmacologically active amounts of adenosine are released into the coronary vasculature from Adenowires, as manifested by a significant increase in coronary blood flow during 40 minutes of wire insertion. Adenowires do not cause systemic haemodynamic effects nor precipitate heart block. Scanning electron micrographs reveal that Adenowires have a smooth surface with a thickness of four to six microns and that, following insertion into the porcine coronary vasculature for 40 minutes, Adenowires do not differ from conventional wires with regard to the amount of cellular or fibrin deposition on the wire’s surface. We conclude that Adenowires represent a novel method for maintaining microcirculatory blood flow during PCI procedures.
Cardiac interventionalists are increasingly acknowledging that the occurrence of MVO has an important negative impact on outcomes2-6. MVO manifests in 4-8% of elective procedures and in more than 50% of STEMIs, where MVO associates with larger infarct size, abnormal ventricular remodelling, arrhythmias and congestive heart failure3,4,7,8. MVO is an important predictor of five-year mortality independent of infarct size10.
The realisation that prevention and prompt reversal of MVO are paramount to improve outcomes in high-risk PCI is motivating the evaluation of numerous devices and pharmacologic approaches to ameliorate MVO. However, studies indicate that various mechanical thrombectomy and embolic protection devices fail to improve indices of tissue perfusion, infarct size or mortality during PCI of native coronary vessels21-24.
As a potent arteriolar vasodilator, adenosine rapidly reverses the effects of elevated levels of powerful vasoconstrictors in the microcirculation after PCI3,4,11,12. Adenosine’s antiplatelet effects and its ability to restore profibrinolytic activity of endothelial cells inhibit thrombus formation and embolisation3,4, and endogenous adenosine inhibits thromboemboli during low-flow ischaemia in a canine model25. Adenosine also functions as an endogenous cardioprotective agent since it is an important mediator of pre- and post-conditioning4. Experimental and clinical studies show that adenosine infusions of one to three hours significantly improve myocardial salvage and tissue blood flow4,13,14,26. The AMISTAD studies show that a three-hour intravenous infusion of adenosine results in significant decreases in infarct size in anterior STEMI patients undergoing reperfusion strategies3,4,14. Other studies demonstrate that intracoronary infusions or boluses of adenosine prevent myocardial necrosis during routine PCI and reverse acute no-reflow in stable and STEMI patients and in patients with SVG27-31.
There are several reasons for developing an adenosine-eluting wire (Adenowire) for targeted delivery of the nucleoside to improve post-PCI tissue perfusion. First, since the guidewire is the first mandatory device placed for a PCI procedure, Adenowires would not require any additional manipulations by the interventionalist. Second, Adenowires would allow the immediate, continuous and concentrated administration of adenosine to the microvascular bed distal to the culprit lesion. Third, Adenowires would premedicate the vascular tree prior to establishment of epicardial flow in STEMI procedures, thereby preconditioning the myocardium from reperfusion injury. Fourth, Adenowires would allow for high local adenosine concentrations, thereby optimising cardioprotection while avoiding side effects with high-dose systemic administration of adenosine. Finally, numerous clinical studies indicate that, because of the multiple mechanisms whereby adenosine attenuates MVO and cellular injury during ischaemia and reperfusion, adenosine is a superior candidate to other pharmacologic agents for MVO.
In conclusion, utilising polyurethane technology, we have developed a non-toxic pentameric form of adenosine that can be coated onto guidewires. This composition allows for continuous elution of adenosine, likely via hydrolysis of the pentamer, into the distal vascular bed during PCI. Since the guidewire is the first device to cross the lesion it would prophylactically medicate the bed with high concentrations of drug at the target site and would avoid the need for additional considerations. These experimental data support the rationale for the use of Adenowire in further studies in patients with coronary artery disease undergoing PCI to determine its ability to reverse NRP and prevent MVO. Since adenosine is the preferred agent to measure FFR to evaluate severity of epicardial stenotic lesions, application of PA to a pressure wire may prove to be another useful clinical application.
Funding
This project was supported by Grant Number R41HL102891 from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
Conflict of interest statement
M. Forman, J. Zhang, Z. Mi and E. Jackson are listed as inventors on the Adenowire pending patent. The other authors have no conflicts of interest to declare.

