Abstract
Transcatheter valve interventions have transformed the outcomes of patients with valvular heart disease who are at high risk for surgery. With the increasing utilisation and expansion of transcatheter valve interventions, it is of utmost importance to be familiar with their potential complications and their subsequent management, especially given the relative infrequency of many of these issues in contemporary practice. Herein, we present a State-of-the-Art review article focusing on the complications, their prevention, and treatment following transcatheter aortic valve implantation, mitral transcatheter edge-to-edge repair, and transcatheter mitral valve replacement.
Transcatheter valve interventions have transformed the outcomes of patients with valvular heart disease at all levels of surgical risk1. Herein, we present a State-of-the-Art overview on the strategies for prevention and management of complications following valvular interventions with a focus on transcatheter aortic valve implantation (TAVI), mitral transcatheter edge-to-edge repair (M-TEER), and transcatheter mitral valve replacement (TMVR).
Transcatheter aortic valve implantation
TAVI has become the standard treatment option for patients with severe symptomatic aortic stenosis (AS) across the whole spectrum of risk. With the expansion of TAVI to lower-risk, younger patients2345 and the consequent dramatic increase in the number of procedures6, it is important to recognise the complications of TAVI and understand their management. Below, we discuss the important complications following TAVI and their subsequent management (Table 1, Central illustration).
Table 1. Summary of complications after TAVI, prevention strategies and their subsequent treatment.
| Entête ajoutée | ||
|---|---|---|
| Complications following TAVI | Prevention strategies | Treatment strategies |
| Stroke | Cerebral embolic protection devicesIn patients on OAC, continuation of OAC rather than interruption of OAC periprocedurallyHeparin administration during the procedure to be ACT guided between 250 and 300 secPost-TAVI antithrombotic therapy:SAPT preferred in patients without an indication for OACContinue OAC regimen without adding antiplatelets in patients with an indication for OAC | Options available include conservative management vs thrombolytics vs mechanical thrombectomy |
| Conduction disturbances | Consider the use of balloon-expandable valves, especially in patients with pre-existing conduction abnormalitiesHigh valve deployment using the cusp overlap techniqueImplanting the valve at a depth less than the membranous septum length | Rapid atrial pacing at end of procedure to risk-stratify patientsPermanent pacemaker if needed |
| Vascular complications | Preprocedural MDCTReal-time ultrasound guidance and use of fluoroscopyUse of micropuncture kitUse of peripheral intravascular lithotripsy in patients with severe iliofemoral calcific stenosis to facilitate transfemoral accessUse of vascular closure devices | Balloon angioplastySurgical repair |
| Paravalvular regurgitation | Preprocedural CTConsider newer-generation valves which have an additional sealing skirt | Ventricular pacing at high ratesBalloon dilatationValve-in-valve with second prosthesisPercutaneous paravalvular leak closure plugs |
| Annular rupture | In patients with high-risk anatomical features, self-expanding valves are preferredIf a balloon-expandable valve is necessary, then underfilling is encouragedHigher valve implantation in cases of severe LVOT calcification | Contained rupture: conservative approach, pericardiocentesisUncontained rupture: emergent surgery |
| Coronary obstruction | Use of partially or fully recapturable transcatheter valveProphylactic coronary protectionBASILICA | Immediate cannulation of affected coronary arteryHaemodynamic supportEmergent surgery if needed |
| Acute kidney injury | PrehydrationContrast-sparing strategiesLow-contrast volume CT protocolsAvoid nephrotoxic medications | |
| ACT: activated clotting time; BASILICA: Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction; CT: computed tomography; LVOT: left ventricular outflow tract; MDCT: multidetector computed tomography; OAC: oral anticoagulation; SAPT: single antiplatelet therapy; TAVI: transcatheter aortic valve implantation | ||
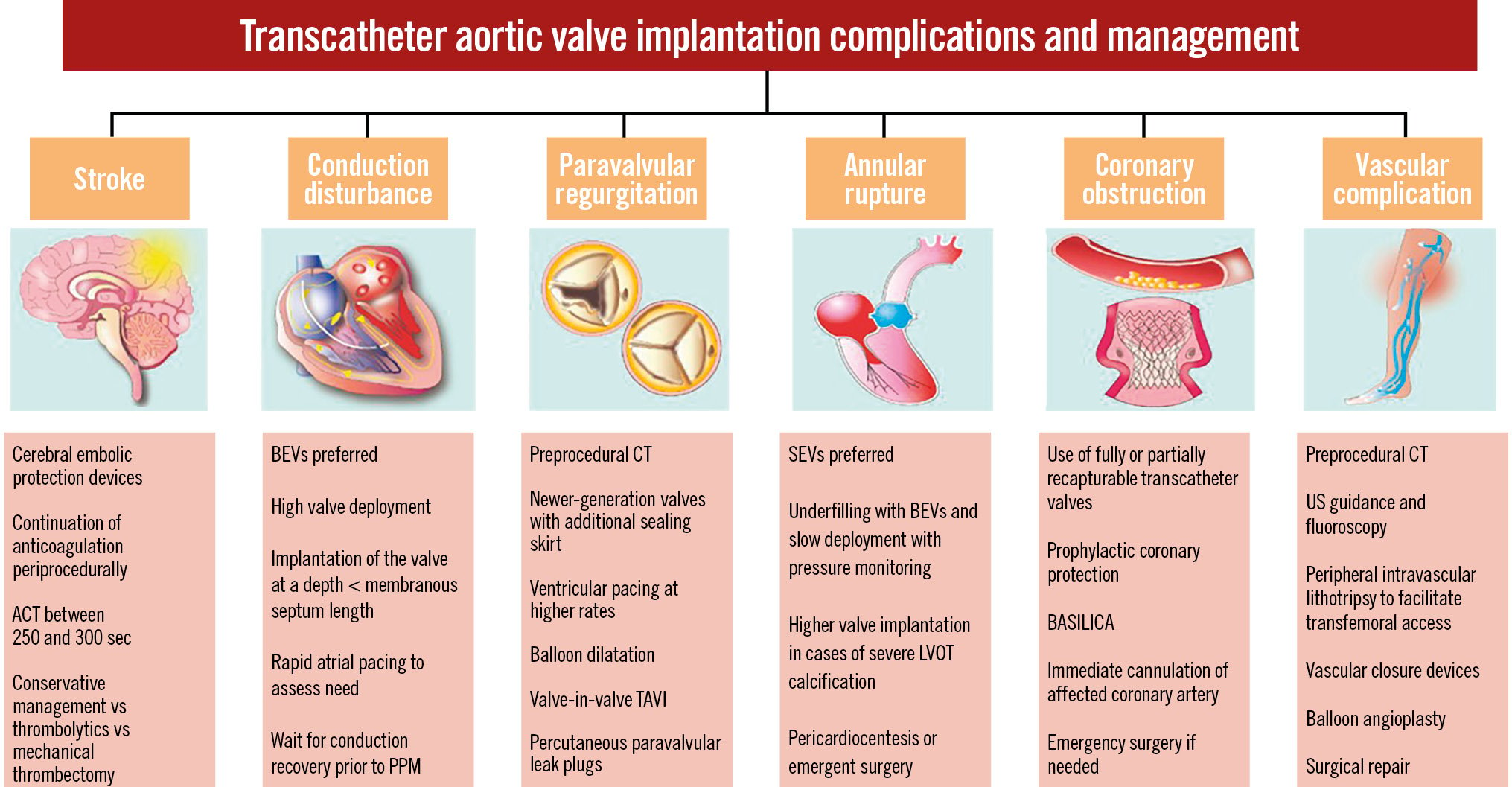
Central illustration. Transcatheter aortic valve implantation complications and their management. ACT: activated clotting time; BASILICA: Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary
Artery obstruction; BEV: balloon-expandable valve; CT: computed tomography; LVOT: left ventricular outflow tract;
PPM: permanent pacemaker; SEV: self-expanding valve; TAVI: transcatheter aortic valve implantation; US: ultrasound
Stroke
Postprocedural stroke remains one of the most dreaded complications following TAVI. Important risk factors for stroke after TAVI include a history of cerebrovascular disease, advanced age, peripheral vascular disease, atrial fibrillation, longer procedure duration, and the need for balloon post-dilatation78. An analysis of more than 100,000 patients from the Society of Thoracic Surgeons (STS)/American College of Cardiology Transcatheter Valve Therapy (TVT) Registry reported an overall stroke incidence of 2.3% after TAVI in a real-world patient population, which remained largely unchanged from 2011 to 2017. The occurrence of stroke was associated with a 6-fold increase in 30-day mortality following TAVI9.
Prevention of stroke
The majority of post-TAVI strokes occur during the acute periprocedural phase (~72 hours) and are due to embolisation of debris from the valve or the vasculature10. Cerebral embolic protection (CEP) devices (CEPDs) are designed to capture and/or deflect the debris and thereby intuitively reduce the incidence of periprocedural stroke. Currently, the only CEPD approved by the U.S. Food and Drug Administration (FDA) is the SENTINEL CEP (Boston Scientific)11. Use of the SENTINEL CEP during TAVI has been evaluated in single-centre studies12, the TVT Registry13, administrative databases14, and one randomised clinical trial1516. PROTECTED TAVR16 was a large, randomised, prospective trial of 3,000 patients which demonstrated that there were no significant differences in the incidence of periprocedural stroke within 72 hours after TAVI between the CEP and control groups (2.3% vs 2.9%, difference –0.6 percentage points, 95% confidence interval: –1.7 to 0.5; p=0.30). Although the trial was not powered to assess disabling stroke, the incidence was lower in the SENTINEL CEP group (0.5% vs 1.3%). An analysis from a nationwide database14 demonstrated that mortality after stroke was significantly lower amongst patients who underwent TAVI with CEP than those without it (6.6% vs 11.8%; p=0.02). Additional data on the effectiveness of CEP during TAVI are forthcoming from ongoing trials, in particular, the British Heart Foundation Randomised Trial of Routine Cerebral Embolic Protection in Transcatheter Aortic Valve Implantation (BHF PROTECT-TAVI; ISRCTN Registry number: ISRCTN16665769), which has a projected enrolment of nearly 8,000 patients. In addition to the SENTINEL CEP, the TriGUARD device (Keystone Heart) is another cerebral protection system which has European Conformity (CE) approval. Table 2 summarises the evidence to date of the safety and efficacy of various CEPD systems used in the TAVI population.
Other considerations relevant to periprocedural cerebrovascular events relate to weighing the competing risks of thrombosis and bleeding, including pharmacotherapy and periprocedural management of anticoagulation, as well as adjunctive device therapies. One study evaluated the safety and efficacy of periprocedural continuation versus interruption of anticoagulation after TAVI17, demonstrating lower risks of stroke and blood transfusion in the continuation group. Heparin administration to reduce periprocedural thrombosis is generally guided by the activated clotting time (ACT), aiming for between 250 and 300 s. At the end of the procedure, protamine is frequently given for heparin reversal, and it has been shown to reduce the rates of life-threatening and major bleeding without increases in the occurrence of stroke and myocardial infarction18. Whether heparin reversal may increase periprocedural cerebrovascular events in patients with a high baseline stroke risk remains unclear presently.
Table 2. Summary of various different cerebral embolic protection devices and their studies.
| Entête ajoutée | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Device | SENTINELa | TriGUARD 3b | ProtEmboc | Emblokd | Embolinere | |||||
| Cerebral embolic protection | Partial | Complete | Complete | Complete | Complete | |||||
| Regulatory status | CE, FDA | CE | SIH | SIH | SIH | |||||
| Vascular access | Right radial | Femoral | Left radial | Femoral | Femoral | |||||
| Study name | MISTRAL-C107 | CLEAN-TAVI | SENTINEL | PROTECTED TAVR | DEFLECT III | REFLECT II108 | PROTEMBO SF TRIAL (FIH) | PROTEMBO C TRIAL | Latib A et al | SafePass clinical programme109110 |
| NCT reference | - | NCT01833052 | NCT02214277 | NCT04149535 | NCT02070731 | - | NCT03325283 | NCT04618718 | NCT03130491 | - |
| Study design | RCT | RCT | RCT | RCT | RCT | RCT | Non-RCT | Non-RCT | Non-RCT | Non-RCT |
| Number of patients | 65(device arm: 32; control arm: 33) | 100(device arm: 50; control arm: 50) | 363(device safety arm: 123; device imaging arm: 121; control arm: 119) | 3,000(device arm: 1,500; control arm: 1,500) | 85(device arm: 46; control arm: 39) | 220(device arm: 162 [41 roll-ins plus 121 randomised]; control arm: 58) | 4 | 41 | 20 | 63(series of 3 single-arm feasibility studies: SafePass FIH: 13; SafePass 2: 31; SafePass 3: 19) |
| Device success | 93.0% | 92.0% | 94.4% | 94.4% | 88.9% | 71.0% | 100% | 94.6% | 100% | 100% |
| Stroke/TIA | Device arm: 0 patients; control arm: 2 patients within 30 days (disabling) | Device arm: 10%; control arm: 10% within 7 days (all non-disabling) | Device arm: 5.6%; control arm: 9.1% within 30 days (p=0.25) | Device arm: 2.3%; control arm: 2.9% within 72 hours (p=0.30) | Device arm: 2.2%; control arm: 5.1% within 72 hours (p=0.30) | Device arm: 6.4%; control arm: 5.3% in hospital (p=1.000) | 0% at 30 days | 1 (2.7%) patient (CEPD retrieved prematurely because of interaction with the TAVI catheter) | 0% at 30 days | 2 (6.5%) patients (at day 1 and at day 17 post-TAVI) in SafePass 2, and 1 (5.2%) patient in SafePass 3 (still to be adjudicated) |
| Brain MRI | New brain lesions at 5-7 days: 78% (73% vs 87%; p=0.31) | New brain lesions at 2 days: 98%Median new lesion number: 4 (IQR 3.3-7.25) vs 10 (IQR 6.75-17.00) (p<0.001) | Median total new lesion volume at 2-7 days: device arm: 102 mm3; control arm: 177 mm3 (p=0.33) | Not performed | New brain lesions at 30 days: 80.8%; device arm: 73.1%; control arm: 88.5% (per-treatment analysis) | Median total new lesion volume at 2-5 days: device arm: 215.39 mm3; control arm: 188.09 mm3 (p=0.405) | 87% reduction of new lesions for protected vs unprotected TAVI at 30 days | Median number of new lesions at 2-7 days: 8 (IQR 3-16) | New brain lesions at2-5 days: 95%Mediannumber of new lesions:10 (IQR 4.75-15.25) | Not performed |
| aBy Boston Scientific; bby Keystone Heart; cby Protembis GmbH; dby Innovative Cardiovascular Solutions; eby Emboline. CE: European Conformity; CEPD: cerebral embolic protection device; FDA: U.S. Food and Drug Administration; FIH: first-in-human; IQR: interquartile range; MRI: magnetic resonance imaging; RCT: randomised controlled trial; SIH: Southern Illinois Healthcare; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack | ||||||||||
Treatment of acute ischaemic stroke after TAVI
The data on emergency management of post-TAVI acute ischaemic stroke are extremely limited. The outcomes of 1,135 patients with acute ischaemic stroke after TAVI were reported using the Vizient Clinical Data Base19. Among these, the majority underwent conservative management (N=1,031, 90.2%), 4.8% (N=54) thrombolytics or 4.4% (N=50) mechanical thrombectomy. In-hospital mortality was 7.7%, 13.0% and 22.0% in the conservative, thrombolytic therapy, and mechanical thrombectomy groups, respectively. The authors concluded that higher mortality rates in the intervention groups were likely from selection bias. Further, Levi et al20 used an international multicentre registry to compare the outcomes of conservative management versus neurointervention for stroke after TAVI. Although the number of patients was too small for conclusive findings, neurointervention (including mechanical thrombectomy or thrombolysis) was associated with 3-fold higher odds of disability-free survival at 90 days compared to conservative management. Because the benefit of neurointervention is highly dependent on the time from the onset of symptoms (i.e., “time is brain”), early identification of stroke symptoms is of the utmost importance21. Utilising conscious sedation as compared with general anaesthesia aids in assessing an awake patient’s responses and may enable earlier detection of stroke22. In Figure 1, we have presented a suggested algorithm for the management of post-TAVI stroke.
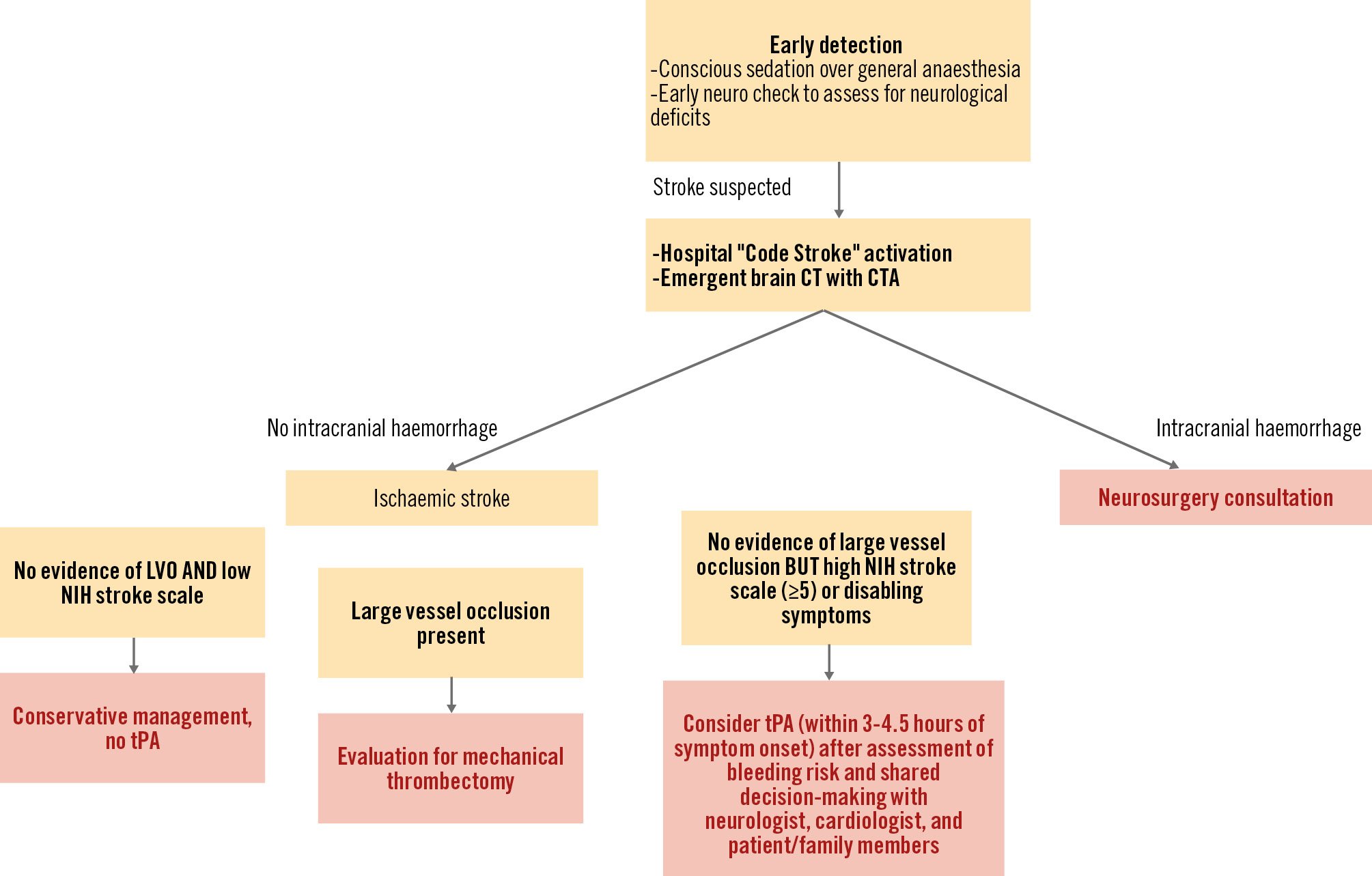
Figure 1. Proposed algorithm for the management of post-TAVI stroke. CT: computed tomography, CTA: computed tomography angiography; LVO: large vessel occlusion; NIH: National Institutes of Health; TAVI: transcatheter aortic valve implantation; tPA: tissue plasminogen activator; US ultrasound
Conduction disturbances
Conduction abnormalities requiring permanent pacemaker (PPM) implantation and the development of new left bundle branch block (LBBB) remain the most common TAVI complications23. Figure 2 represents the anatomical relationship between the fascicles of the left bundle branch and the aortic valve (AV) leaflets, demonstrating that the superior fascicle of the left bundle branch descends at the nadir of the right coronary leaflet24. The incidence of PPM implantation after TAVI with new-generation valves ranges widely, from 2.3% to 36.1%25. The strongest electrocardiographic (ECG) predictor for post-TAVI PPM implantation is the presence of pre-TAVI right bundle branch block (RBBB)26. Other ECG risk factors include left anterior hemiblock, first degree atrioventricular (AV) block and LBBB. With regard to valve choice, the incidences of new periprocedural LBBB and PPM requirement are higher with self-expanding (SEV) compared with balloon-expandable valves (BEVs). Among anatomical predictors, the presence of calcification below the aortic annulus and in the left ventricular (LV) outflow tract (LVOT), a lower valve implantation depth, and a shorter length of the membranous septum (distance between the AV annular plane and the bundle of His) are important predictors of new PPM requirement after TAVI25. Further, patients who receive a valve-in-valve (ViV) have lower rates of PPM implantation, likely due to the rigid structure of the surgical valve that limits compression of the conduction system27. This is likely not the case for redo-TAVI, where the index TAVI frame is relatively compliant27.
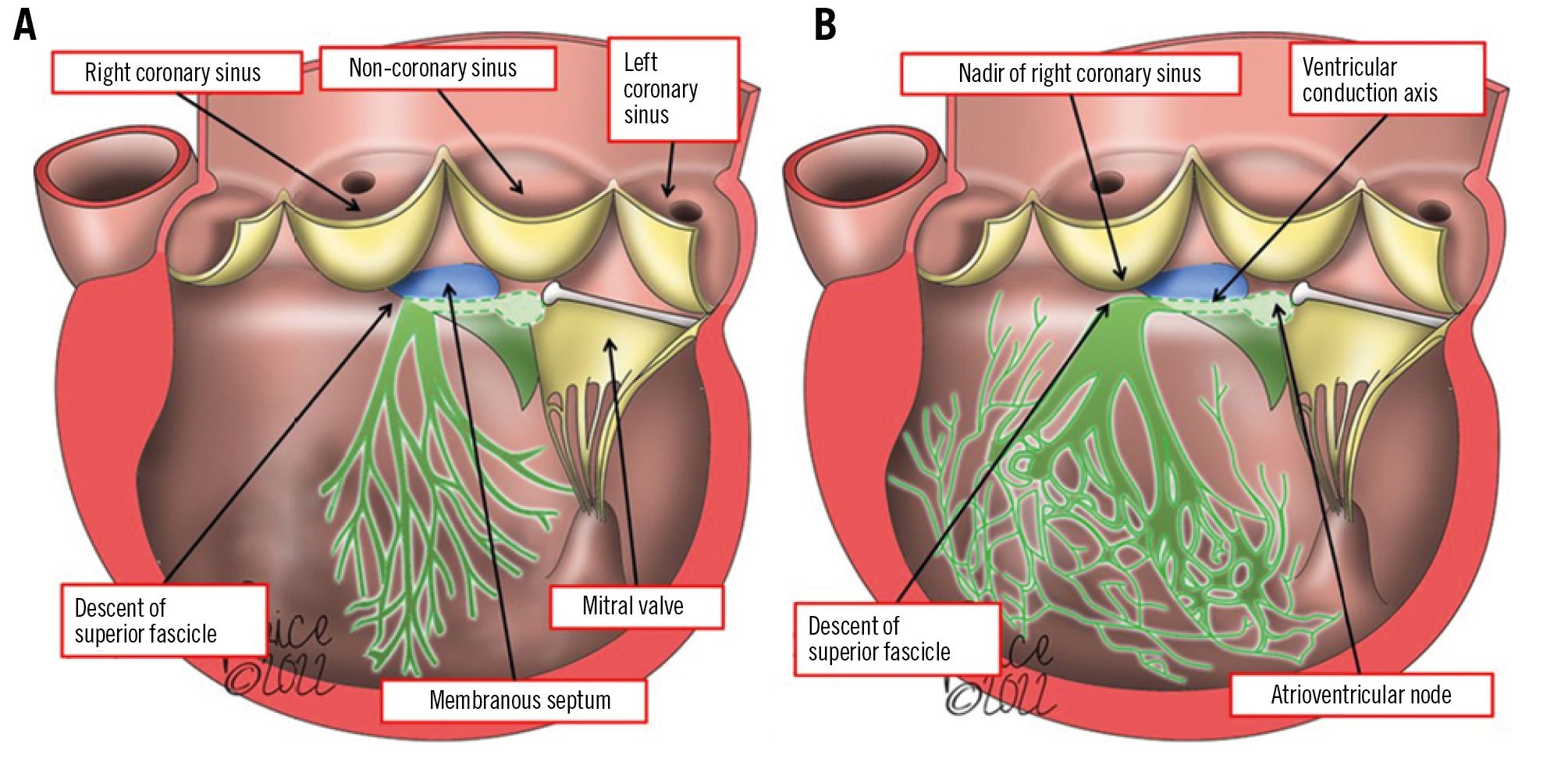
Figure 2. Conduction system anatomy. A) The drawing shows the common, incorrect depiction of the presumed location of the left bundle branch relative to the aortic root. B) The drawing represents the correct location, showing the superior fascicle of the left bundle branch descending at the nadir of the right coronary leaflet of the aortic valve. Adapted with permission from24.
Prevention of conduction abnormalities
Given the higher risk of PPM implantation with self-expanding valves28, it may be reasonable, especially in patients with pre-existing RBBB, to consider a balloon-expandable valve. If an SEV is deemed necessary because of local expertise or other anatomical factors, it should be noted that the latest-generation SEVs, such as the ACURATE neo2 (Boston Scientific), with its lower radial strength, have been associated with relatively low PPM implantation rates29. Further, procedural techniques have been developed with an aim to achieve a higher implantation depth with both BEVs and SEVs.
The high deployment technique (HDT) using the SAPIEN S3 BEV (Edwards Lifesciences) to isolate the non-coronary cusp (NCC) and minimise implantation depth has been associated with significantly reduced PPM implantation rates (5.5% vs 13.1%; p<0.001)30. This system, also called the “cusp overlap” technique31, involves overlapping the left and right coronary cusps to isolate the non-coronary cusp. This is achieved by rotating the C-arm in a right anterior oblique (RAO) caudal direction; the optimal projection can be determined from preprocedural computed tomography (CT) or achieved at the time of the procedure by “squaring off” any parallax in the transcatheter heart valve (THV) stent frame. The key advantage of this projection is that both the delivery catheter and aortic cusps can be aligned, delivery catheter parallax is eliminated, and the LVOT is not foreshortened (as it is in a left oblique anterior view), which allows for a more precise and higher implantation depth. The above technique has also demonstrated a reduction in PPM implantation with SEVs32. Additionally, implanting the valve at a depth less than the infra-annular membranous septum length (as measured by CT) was also shown in the MIDAS study to reduce the rates of PPM implantation from 9.7% to 3.0% and of new onset LBBB from 25.8% to 9.0%33. Of note, there exist risk scores, including the PRIME34 and Emory risk scores (validated for BEVs only)35, for predicting pacemaker implantation following TAVI.
Treatment of conduction abnormalities
At our institution, we routinely perform a rapid right atrial (RA) pacing study after valve implantation by withdrawing the temporary pacemaker lead to the RA and pacing from 70 to 120 beats/min (at 10 beats/min increments) at the conclusion of the procedure to risk-stratify patients36. Those who develop Wenckebach with atrial pacing, especially at lower rates, as well as patients with pre-existing atrial fibrillation (in whom the rapid atrial pacing study is not possible), and those who develop postprocedural high-grade AV block (AVB) may require an individualised approach while keeping in mind the periprocedural ECG changes, anatomical factors, and other variables that can guide the decision to continue inpatient versus outpatient rhythm monitoring (Figure 3). In our study of 284 TAVI patients, 130 (46%) developed pacing-induced Wenckebach. Among the group who did not (n=154, 54%), PPM implantation was required in only 1.3% of patients, with a negative predictive value of 98.7%.
An expert panel proposed an algorithm for the management of conduction disturbances post-TAVI37. The following recommendations were made based on ECG changes during/post-TAVI and preprocedural conduction disturbances:
Group 1 – no ECG changes and no evidence of RBBB preprocedure: it is recommended to remove the temporary pacing wire and to maintain continuous telemetry for 24 hours. If there is no evidence of bradyarrhythmias or conduction disturbances, patients can be safely discharged.
Group 2 – no ECG changes, but with evidence of pre-existing RBBB: it is recommended that the temporary pacing wire be maintained for 24 hours. If there is evidence of high-grade AVB (HAVB) or complete heart block (CHB), then PPM implantation is recommended. If no ECG changes or significant bradyarrhythmias occur within the 2 to 3 days following the procedure, the patient can probably be safely discharged.
Group 3 – ECG changes in the form of a persistent increase in PR interval or a QRS duration >20 ms in patients with pre-existing conduction disturbances (RBBB, LBBB, intraventricular conduction delay [IVCD] with QRS duration >120 ms, 1st-degree AVB): if there is a regression of ECG changes to baseline values or there are no further ECG changes and the QRS duration <150 ms and PR interval <240 ms, then the patient can be discharged with no PPM implantation at 1-2 days post-TAVI. If at 24 h post-TAVI, the PR and QRS intervals remain stable but >240 ms or >150 ms, respectively, and ≥20 ms longer than baseline, it is recommended to maintain the temporary pacing wire for another 24 h. If no decrease in the PR interval or QRS duration occurs at day 2, the patient can be considered at risk for more advanced conduction disturbances requiring PPM implantation (HAVB/CHB).
Group 4 – new-onset LBBB after TAVI: it is recommended to maintain the temporary pacing wire for 24 hours. Earlier removal of the temporary pacing wire can be considered if LBBB resolves in <24 hours. If LBBB persists but there is no further progression of the duration of the QRS or PR interval, temporary pacing can be discontinued, and if no further ECG changes are observed up to day 2 to 3 post-TAVI, the patient can be discharged. These patients, however, are at an increased risk of delayed HAVB/CHB requiring PPM implantation. If further prolongation of the QRS duration or PR interval (of at least 20 ms) is observed at day 1, the temporary pacing wire is recommended to be maintained for an additional 24 h. If the prolongation of the QRS duration or PR interval continues at day 2, direct PPM implantation can be considered. If no further prolongation of the QRS duration or PR interval is observed at day 2, temporary pacing can be discontinued and the patient can remain hospitalised for 1 additional day (with daily ECG and telemetry). If no further changes are observed, the patient can be discharged at day 3 post-TAVI. These patients, however, are at increased risk of delayed HAVB/CHB requiring PPM implantation. Utilising continuous ECG monitoring (minimum of 2 to 4 weeks) and/or electrophysiology (EP) studies may be considered.
Group 5 – HAVB/CHB during the procedure: it is recommended to maintain the temporary pacing wire in patients with procedural persistent HAVB/CHB and monitor the patients in an intensive care unit. If HAVB/CHB persists at 24 h post-TAVI, a PPM is recommended to be implanted. If HAVB/CHB recovers the day after TAVI, the temporary pacing wire can be removed and the patient can remain hospitalised for 1 additional day, with telemetry and daily ECG. If another episode of HAVB/CHB occurs, PPM implantation is recommended.
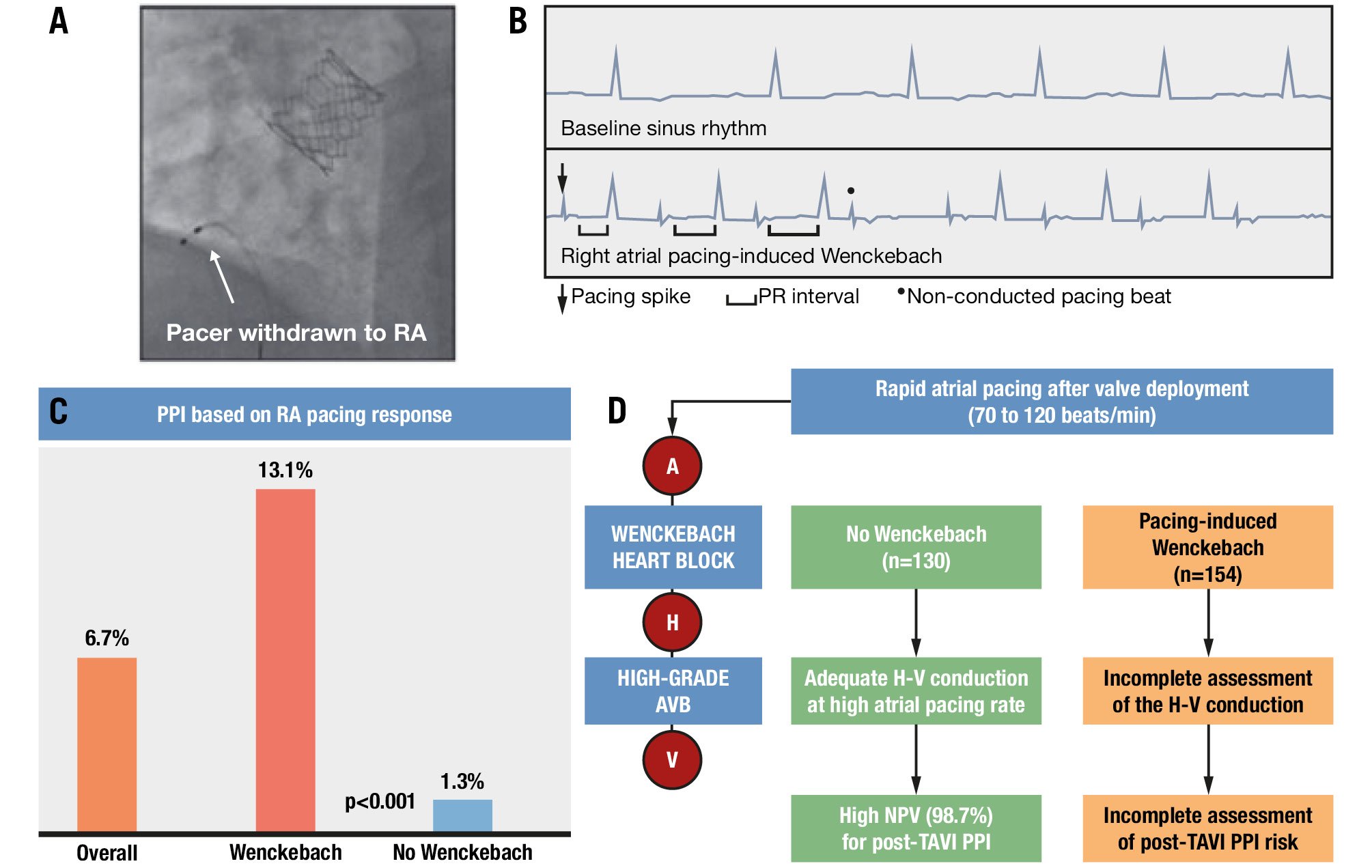
Figure 3. Rapid pacing of the right atrium in sinus rhythm. If the AV node conducts 1:1 with the right atrial pacing at 120 beats/min, then there is a less than 2% chance of requiring a permanent pacemaker. A) Pacer withdrawn to the right atrium; (B) right atrial pacing-induced Wenckebach; (C) permanent pacemaker implantation rates based on right atrial pacing response; (D) rapid atrial pacing after valve deployment. AV: atrioventricular; AVB: atrioventricular block; H: His; NPV: negative predictive value; PPI: permanent pacemaker implantation; RA: right atrium; TAVI: transcatheter aortic valve implantation; V: ventricle
Vascular complications
In early TAVI clinical trials with first-generation devices and 18-24 Fr sheath delivery systems, vascular complications were reported in nearly 15% of patients3839. Over time, there has been a significant reduction in major vascular complications, with an incidence of 6% to 8% in recent TAVI trials40. This reduction in incidence has been driven by a combination of improved technologies with lower-profile delivery systems, decreasing patient risk profiles, multidetector CT (MDCT) assessment of peripheral vasculature, increased operator experience and improved technique41. Vascular complications and bleeding events pose a significant challenge in contemporary practice and are associated with increased mortality and prolonged hospitalisation40. Vascular complications most commonly occur at the access site, and studies consistently show that failure of the vascular closure device (VCD) is the most common cause of a major vascular complication. A number of patient- and procedure-related factors have been identified as increasing the incidence of vascular complications. Patient-related factors include female sex, elderly age, obesity, peripheral vascular disease, circumferential calcification, and vascular tortuosity. Procedure-related factors include larger sheath sizes and increased sheath-to-femoral artery ratio42.
Prevention of vascular complications
A detailed evaluation of the peripheral vessels using preprocedural MDCT is critical for reducing the risk of vascular complications. The role of MDCT is to assess the minimal luminal diameters of iliac and femoral vessels, iliofemoral vessel tortuosity, vessel calcification location, location of femoral bifurcation, and the presence of any additional vascular pathology41. In patients with significant anterior calcification or deep femoral arteries, surgical cutdown may be preferable to percutaneous access to avoid the increased risk of VCD failure. When feasible, transradial access can be considered as an alternative to the contralateral diagnostic/angiographic catheter placement; this approach may be associated with a significant reduction in vascular complications compared to a conventional bifemoral approach43. Preprocedural CT may be employed to determine the optimal site for arterial puncture. Use of real-time ultrasound guidance is increasing and is associated with a reduction in the incidence of vascular complications. Fluoroscopy can also be used to facilitate femoral puncture, with needle insertion performed under active X-ray imaging given an understanding of the common femoral artery (CFA) anatomy that is based on preprocedural imaging as well as avoidance of fluoroscopic calcification44.
At the end of the procedure, the closure of large arteriotomies can be safely and effectively achieved using VCDs45. Their use is associated with reductions in procedural time, hospital stay, and complication rates. However, VCD failure is still the leading cause of major vascular complications. For larger arteriotomies, preclosure with one ProStar XL (Abbott), or more recently, two Perclose ProGlides (Abbott), is historically preferred, though our group and others have demonstrated the feasibility and safety of a single Perclose device use in this setting4647. An alternative is the collagen plug-based MANTA device (Teleflex Inc.), which is the only commercially available VCD that is formally FDA approved for large-bore arterial access. However, the recent CHOICE-CLOSURE randomised trial (MANTA vs ProGlide) showed a higher rate of access site or access-related vascular complications with use of this device; the inability to maintain wire access before haemostasis is confirmed is also an important consideration48.
At our institution, unilateral sheath insertion has become the preferred access strategy, with placement of a 5 Fr sheath for aortic root procedures and femoral completion angiography 2-3 cm inferior to the TAVI delivery sheath access point49. With the use of a unilateral access site, complications can be easily managed as this inferior sheath is already across the delivery sheath access point, making balloon dilatation and/or stent placement straightforward in comparison to crossover techniques from the contralateral femoral artery or via the wrist (Figure 4). Furthermore, alternative access TAVI can be utilised in case of a challenging common femoral artery access.
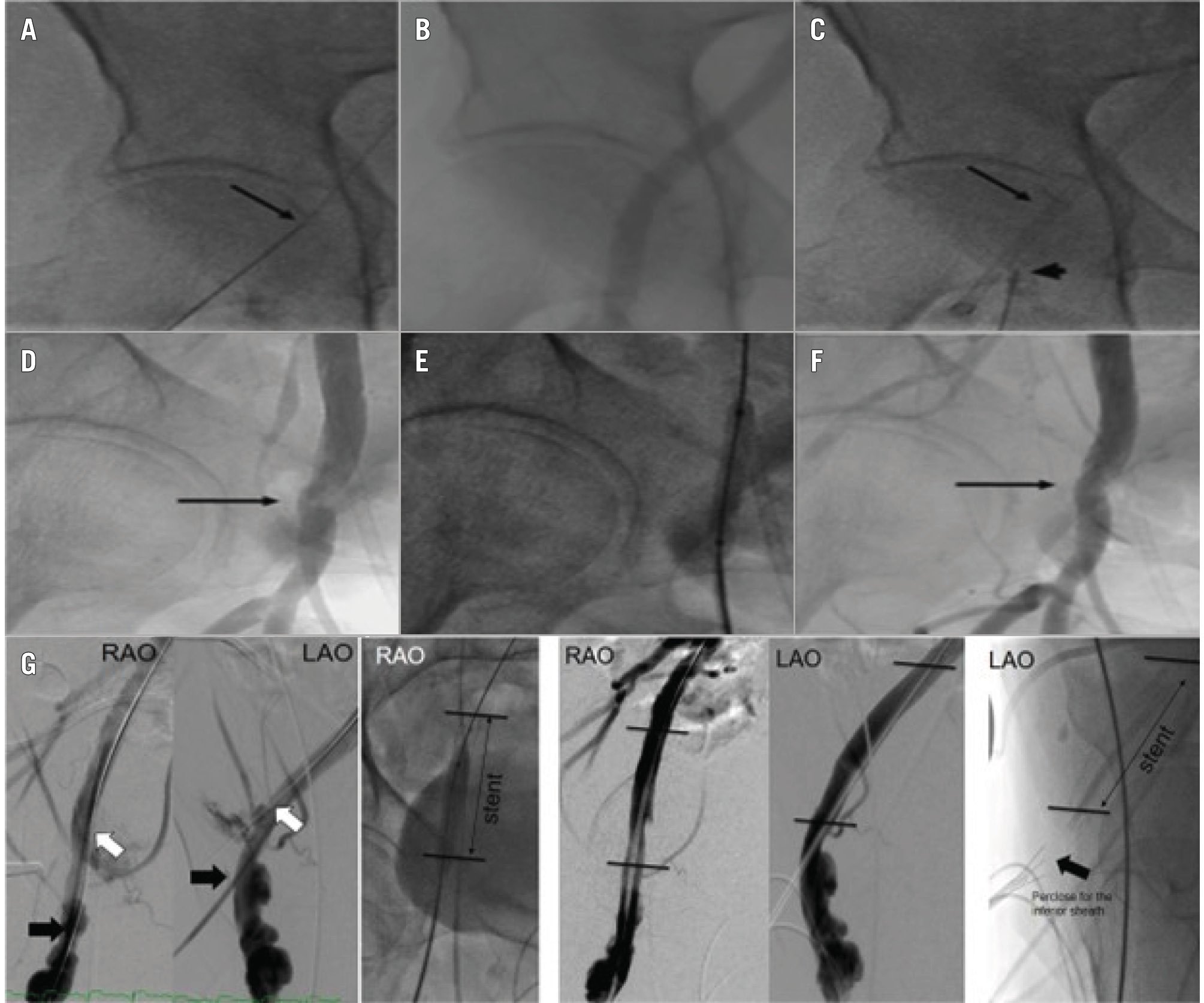
Figure 4. Peripheral intervention via unilateral arterial access. A) Proximal RFA access site for delivery sheath (arrow). B) RFA angiogram. C) Proximal RFA sheath (arrow) and inferior RFA access site (arrowhead). D) Completion angiogram via inferior sheath shows delivery sheath site stenosis (arrow). E) Angioplasty via inferior sheath. F) Resolution of RFA delivery site stenosis (arrow). G) A separate patient with iliofemoral dissection who was successfully treated with a stent via unilateral access. LAO: left anterior oblique; RAO: right anterior oblique; RFA: right femoral artery
Treatment of vascular complications
Below, we summarise the potential vascular complications and their treatment:
1. Iliofemoral dissection: dissections occur most commonly in the external iliac artery. Retrograde or contralateral antegrade angiography after removal of the TAVI delivery sheath is strongly recommended. Asymptomatic small dissections without flow limitation can be treated conservatively. In case of flow limitation, angioplasty with prolonged balloon inflation is the preferred treatment. Extensive dissection may require uncovered or covered stent implantation and is favoured over open surgical treatment50.
2. Iliofemoral rupture: this can be managed with immediate reversal of anticoagulation followed by sealing of the tear either through reintroduction of the sheath or emergency balloon occlusion using the diagnostic catheter sheath (which may need to be upsized). Larger vessel injuries that do not seal may require covered stent implantation and/or surgical repair.
3. Access site bleeding and haematoma: most haematomas can be managed conservatively with manual compression and reversal of anticoagulation. In the case of a large expanding haematoma, a prolonged balloon angioplasty should be performed. If this occurs once the patient is out of the procedural suite, emergency angiography and treatment should not be delayed in the pursuit of confirmatory CT scanning, since haemodynamic compromise can ensue.
4. Pseudoaneurysm: pseudoaneurysms of size <3-3.5 cm close spontaneously in the majority of cases and can just be monitored with serial ultrasound exams. For a size >3.5 cm or an expanding pseudoaneurysm, ultrasound-guided thrombin injection is recommended42. Covered stents may be utilised for pseudoaneurysms.
5. Arterial stenosis, arterial occlusion or thrombosis: stenosis of the CFA occurs primarily after the deployment of a VCD. If severe stenosis is limiting flow, balloon dilatation is helpful and may obviate the need for stent placement. For arterial thrombosis, thrombectomy and/or balloon angioplasty is also useful.
Paravalvular regurgitation
Moderate or greater paravalvular regurgitation (PVR) used to occur in approximately 10-25% of TAVI procedures with early-generation prostheses; however, the widespread adoption of MDCT assessment for optimal valve sizing and procedural planning, and the introduction of newer-generation devices with external sealing skirts has decreased the incidence of PVR to <5%51. Whether even mild PVR is associated with a significant increase in short- and long-term mortality and morbidity is a matter of controversy, though consideration should be given to the mischaracterisation of moderate PVR as only mild if not thoroughly assessed52.
In contemporary practice, aortic regurgitation (AR) is assessed using the following three specific modalities: echocardiography, haemodynamics, and aortography. Although transthoracic and transoesophageal echoÂcarÂdioÂgraphy (TOE) have long been a mainstay for diagnosis of the type and degree of AR, they may sometimes be limited in accuracy and reproducibility5354. However, echocardiography is an important tool for AR assessment, in addition to other potential complications, and is almost indispensable for distinguishing paravalvular versus valvular AR. At our institution, we also place substantial emphasis on haemodynamic assessment in our TAVI procedures, carefully comparing both aortic diastolic and LV end-diastolic pressures (LVEDP) before and after TAVI, the quality of the dicrotic notch after TAVI, slope of the LV diastolic pressure increase, and response of the LVEDP and aortic diastolic pressure to the long RR interval following a premature ventricular contraction (Figure 5). Several prognostically relevant haemodynamic indices have been proposed, with the AR index (calculated as LVEDP–DBP/SBP, in which DBP is the diastolic blood pressure and SBP is the systolic blood pressure) being the most widely adopted5556. Aortography is also an important part of our post-TAVI implantation routine. It allows assessment of coronary flow, confirmation of depth of implantation, insight into annular trauma, and, most importantly, an adjunctive assessment of AR.
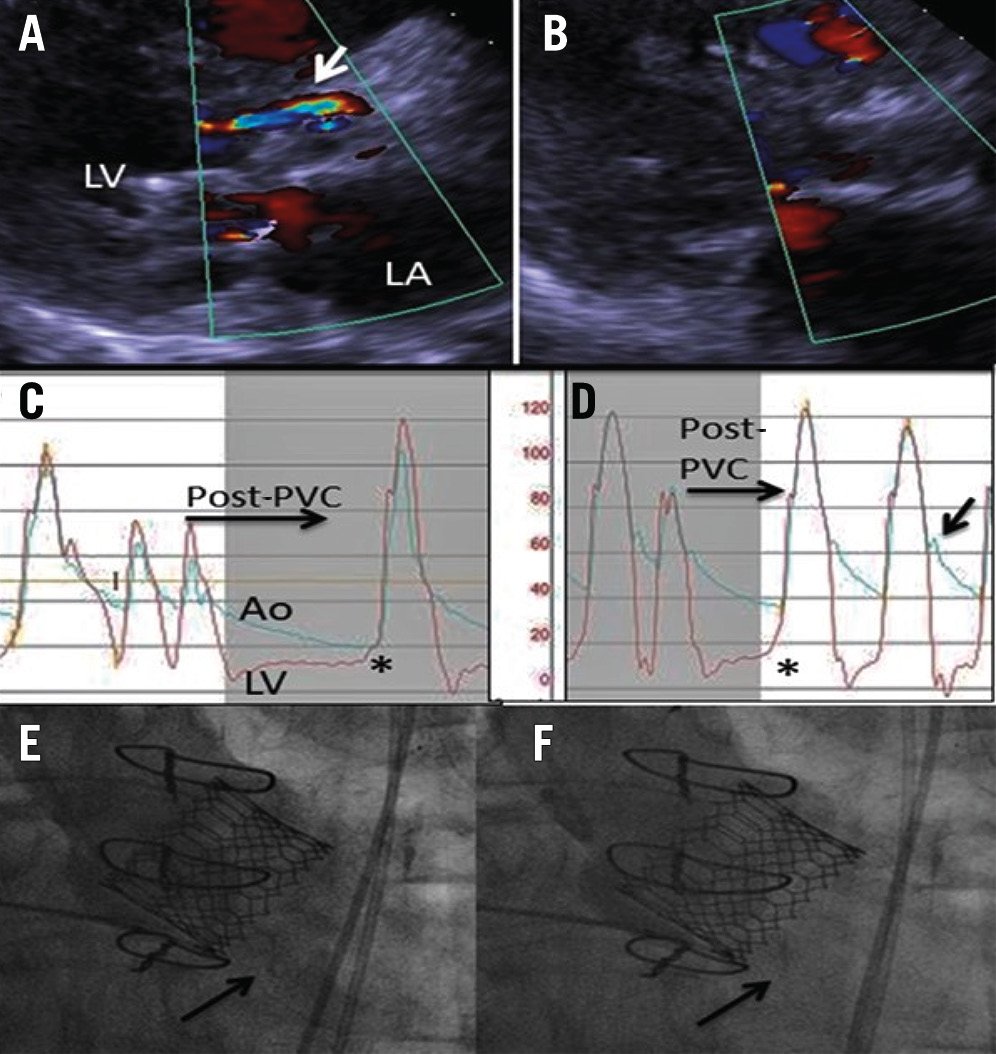
Figure 5. Paravalvular regurgitation before and after post-dilatation. The transthoracic echocardiography demonstrates anterior PVR (arrow) before (A) and after (B) post-dilatation. The haemodynamic evaluation demonstrates the lack of an aortic dicrotic notch, aortic diastolic/LVEDP equalisation with a long RR interval after a PVC (asterisk) before post-dilatation (C), and a prominent dicrotic notch (arrow) and good aortic diastolic/LVEDP separation (asterisk) after post-dilatation (D). The aortogram demonstrates LVOT opacification during diastole (arrow) after TAVI (E) which is corrected by post-dilatation (F). Ao: aorta; LA: left atrium; LV: left ventricle; LVEDP: left ventricular end-diastolic pressure; LVOT: left ventricular outflow tract; PVC: premature ventricular contraction; PVR: paravalvular regurgitation; TAVI: transcatheter aortic valve implantation
Prevention of paravalvular leak
Accurate preprocedural CT analysis should prevent valve underexpansion and can also be used to identify high-risk features such as heavy annular and/or leaflet calcification, which may lead to valve underexpansion or malpositioning. The SAPIEN 3 Ultra (Edwards Lifesciences), Evolut PRO+ (Medtronic), Navitor (Abbott), and ACURATE neo2 valves all have contemporary-design sealing skirts to minimise paravalvular leak (PVL). Therefore, detailed preprocedural CT evaluation combined with appropriate device selection play a key role in minimising PVL57.
Treatment of paravalvular leak
If the paravalvular regurgitation is mild, it can be managed conservatively with periodic follow-up imaging. In patients with moderate-severe or severe PVR, intervention is needed in cases of LV dilatation, heart failure symptoms, or haemolytic anaemia, the latter of which is relatively rare. Surgical aortic valve replacement can be an option; however, the majority of patients with ≥moderate PVR are at high risk for surgery. Transcatheter therapies are often utilised for the management of PVR. The treatment of PVR depends upon the mechanism that is leading to AR. Recognising the main PVR mechanism is therefore critical for choosing the most suitable approach; this can become more complex when there are multiple underlying mechanisms. Irrespective, in the acute setting when a temporising measure is necessary, ventricular pacing at high rates decreases the regurgitant volume. First, PVR can occur due to undersizing of the valve prosthesis. In these cases, balloon dilatation allows for greater valve expansion and reduction of PVL, although it has to be balanced with an increased risk of annular rupture, and hence, caution is required especially in the presence of extensive calcification58. Second, PVR can occur because of suboptimal placement of a prosthesis with incomplete sealing of the annulus by the valve skirt. In these cases, a ViV approach with a second prosthesis can be employed59. Understanding whether the index valve is too high or too low is imperative, as is assurance that coronary flow will be preserved after performing a TAVI-in-TAVI. Third, PVR can occur because of incomplete apposition of the valve stent frame due to calcification of the annulus or native valve leaflets. In these situations, percutaneous paravalvular leak closure plugs can be used60. Figure 6 demonstrates the use of an Amplatzer vascular plug (Abbott) for the management of paravalvular leak in a patient with a prior 29 mm Evolut valve who had symptomatic aortic regurgitation. In a multicentre registry, Landes et al61 reported the outcomes of 201 patients with greater than moderate PVR who underwent redo-TAVI, vascular occluder plug or balloon valvuloplasty: 43% underwent redo-TAVI, 39% underwent placement of a vascular occluder device, and 18% underwent balloon valvuloplasty. There were lower rates of persistent moderate or greater PVR after redo-TAVI than after either of the two other treatment modalities.
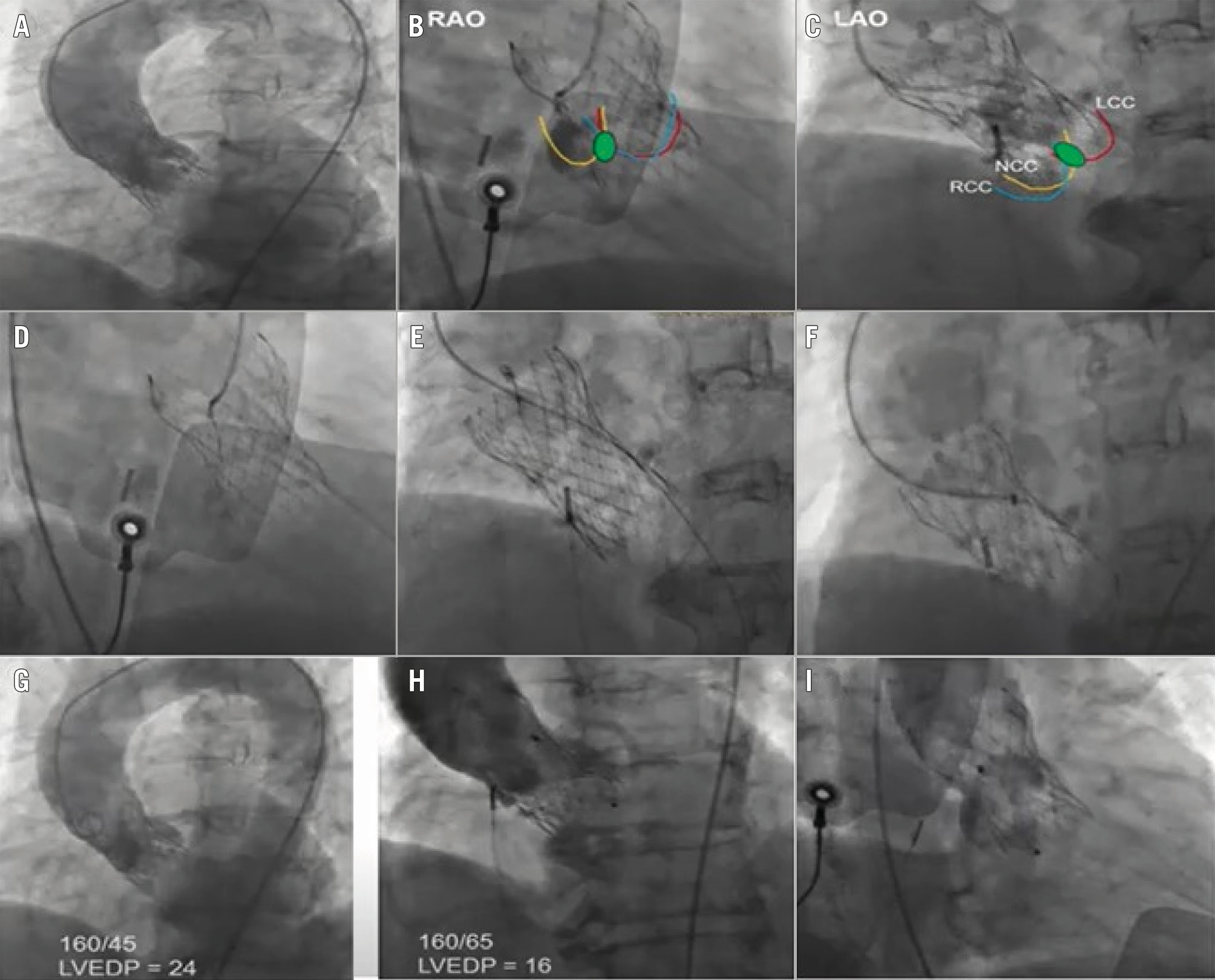
Figure 6. Management of paravalvular leak in a symptomatic 77-year-old female with a prior 29 mm (Medtronic) Evolut valve, using an Amplatzer vascular plug (Abbott). A) Aortogram showing moderate to severe aortic regurgitation; (B, C) localisation of the paravalvular leak in the RAO and LAO views; (D) crossing the leak with a stiff, angled Glidewire (Terumo); (E) confirming the crossing; (F) crossing with a Cook Medical sheath and deployment of a 12 mm AVP II; (G) pre- and (H) post-aortogram with improvement in haemodynamics; (I) final aortogram. AVP: Amplatzer vascular plug; LAO: left anterior oblique; LCC: left coronary cusp; LVEDP: left ventricular end-diastolic pressure; NCC: non-coronary cusp; RAO: right anterior oblique; RCC: right coronary cusp
Annular rupture
Rupture of the AV annulus is a rare complication of TAVI, occurring in <1% of cases. However, it is one of the most feared complications due to the potential rapid onset of haemodynamic collapse and consequent increased mortality rates62. Annular rupture accounts for 15% of patients requiring a bailout surgical procedure following TAVI, and in-hospital mortality is 50% when contained and >75% when uncontained63. Annular rupture includes injuries that occur to the device landing zones (DLZs) encompassing the aortic annulus, aortic sinuses, aortic root and the LVOT. The risk factors for annular rupture include subannular LVOT calcification, BEV oversizing, aggressive balloon post-dilatation, a bicuspid aortic valve, shallow sinuses of Valsalva, and a small aortic annulus (<20 mm)6465.
Prevention of annular rupture
The systematic use of MDCT is critical to minimise the risk of annular rupture during TAVI. A few considerations to mitigate the risk of annular rupture are mentioned below. Valve oversizing (>20%) is a risk factor for rupture; therefore, accurate annular sizing is critical. In cases where MDCT measurements are challenging, balloon sizing can be performed, with aortography used to confirm the absence of aortic regurgitation with fully inflated balloons of known sizes. In patients with high-risk anatomical features, an SEV is preferred, but if a BEV is necessary, then a degree of underfilling to minimise valve oversizing is encouraged. In cases with severe LVOT calcification, a strategy of higher valve implantation can be considered to reduce the radial force of the valve on the LVOT and keep the THV frame at the sinus/commissural level.
Treatment of annular rupture
The management of annular rupture depends on its location and extent. In patients with contained rupture, an initial conservative management strategy is recommended with reversal of anticoagulation, ensuring the availability of blood products and frequent reassessment of clinical status. Contained rupture usually portends a relatively favourable outcome. In uncontained rupture with associated pericardial effusion or tamponade, pericardiocentesis and reversal of systemic anticoagulation is recommended. Autotransfusion of the pericardial drainage can reduce the need for blood products when bleeding is extensive. If the pericardial bleeding cannot be controlled, sternotomy and aortic root repair with or without surgical aortic valve replacement are needed. Surgical techniques for the management of annular rupture include primary repair, patch annulopasty, and aortic root replacement66. In cases where the rupture site can be clearly identified, implantation of a second transcatheter valve can be performed. Tomii et al67 reported the single-centre outcomes of patients with annular rupture after TAVI. Of the 18 patients who experienced annular rupture, 8 were managed conservatively, 5 underwent rescue ViV TAVI, and 5 underwent surgical bailout. Amongst the patients who underwent rescue ViV TAVI, 2/5 converted to surgery. The 30-day mortality rates were highest in the conservative group.
Coronary obstruction
Coronary artery obstruction is a relatively infrequent complication that occurs in <1% of native valve TAVI but is associated with a dismal prognosis and 30-day mortality rates approaching 50% despite attempted rescue revascularisation. The risk of coronary obstruction is higher for ViV TAVI with an incidence of around 2.5%68. TAVI-related coronary obstruction can occur via two mechanisms. Direct coronary obstruction occurs when the transcatheter valve displaces the degenerated native or bioprosthetic valve towards the ostia of the coronary arteries. Indirect coronary obstruction can occur in patients with a narrow sinotubular junction (STJ) by the mechanism of sinus sequestration, i.e., the native or prosthetic leaflets extend from the annulus to the STJ and do not allow flow from the ascending aorta to the coronary sinuses. Coronary obstruction generally presents intraprocedurally with ischaemic ECG changes and commonly involves the left coronary ostium6970.
High-risk anatomical features for coronary obstruction include a low coronary height and narrow sinus of Valsalva diameter. Coronary obstruction is more common during ViV procedures as a consequence of the reduced distance between the valve leaflets and coronary ostia (secondary to the supraâannular design of surgical prostheses) and the narrower sinus of Valsalva (secondary to surgical bioprosthesis suturing). In particular, bioprosthetic valves with leaflets mounting outside an internal stent (e.g., Mitraflow [Sorin Group Inc.] and Trifecta [Abbott]) or stentless bioprosthetic valves are at higher risk because the leaflets of these bioprostheses may extend outward following TAVI deployment. In ViV TAVI patients, a smaller virtual transcatheter heart valve-to-coronary (VTC) distance (≤4 mm) is associated with an increased incidence of coronary obstruction6869707172.
While uncommon, delayed coronary obstruction may also occur during recovery after anticoagulation has reversed and may be due to either development of thrombus in the sinuses around the valve or displacement of leaflet tissue or leaflet calcium73. Postprocedural care teams should therefore be vigilant to assess any patient complaints of chest discomfort, concerning ischaemic changes on ECG, or haemodynamic compromise. Very late sinus sequestration with ensuing coronary ischaemia has also been reported recently following self-expanding TAVI in native AS, although fortunately this appears to be a rare complication74.
Prevention of coronary obstruction
Preprocedural cardiac MDCT is extremely important to identify patients at risk for coronary obstruction. In patients found to be at high risk for obstruction, surgical aortic valve replacement should be considered. If not feasible because of the operative risk of the patient, then multiple strategies can be considered as an alternative. First, the use of a partially or fully recapturable transcatheter valve (Evolut R/PRO, Portico [Abbott]/Navitor) or a valve with a favourable open-cell design (ACURATE neo, Navitor) may be advantageous in patients at high risk for coronary obstruction75, although one should bear in mind that a near-complete deployment of a recapturable device may not reflect the final device positioning on device release.
Second, prior to valve deployment, prophylactic coronary protection with standard 0.014 inch guidewire with or without an undeployed stent can be considered. Following valve deployment, if coronary obstruction ensues, then the stent can be pulled back and deployed in a “chimney” fashion to maintain coronary patency7677. Consideration may also be given to using only a coronary balloon in this setting to avoid a situation in which the coronary is not obstructed but a stent cannot be safely withdrawn without stripping it from the delivery balloon. Further, use of a balloon when necessary may provide timely coronary perfusion while allowing the operator to engage the coronary ostium through the THV frame, allowing both a more coaxial stent deployment as well as a shorter stent length and theoretically easier reaccess to the coronary. When a stent is placed, a stent with a high radial strength should be used, followed by high-pressure post-dilatation, with intravascular ultrasound (IVUS) used to evaluate adequate stent expansion. Given that a portion of the stent protrudes into the aorta and is unlikely to undergo re-endothelialisation, prolonged dual antiplatelet therapy (DAPT) therapy should be considered.
A third strategy is the Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) technique to split either native or bioprosthetic aortic valve leaflets prior to TAVI in order to maintain blood flow into the coronary sinus. The procedure involves intentional laceration of the leaflets using radiofrequency energy delivered to a guidewire suspended between two guiding catheters7879. In the BASILICA trial78, the procedure was successful in 28/30 subjects, and there was 100% freedom from coronary occlusion during TAVI. The key advantage of the BASILICA procedure is the possibility of avoiding stent implantation, which mitigates the need for prolonged DAPT therapy and avoids potential stent-related complications such as underexpansion or restenosis. Typical BASILICA for redo-TAVI has not been proven to be effective, and use of a balloon-assisted (BA-BASILICA) technique is recommended. We have previously reported a novel electrosurgical bailout technique for acute left main occlusion following redo-TAVI in a surgical bioprosthesis (Figure 7)80. Dedicated devices for leaflet splitting to prevent coronary obstruction have been developed and are under investigation81.
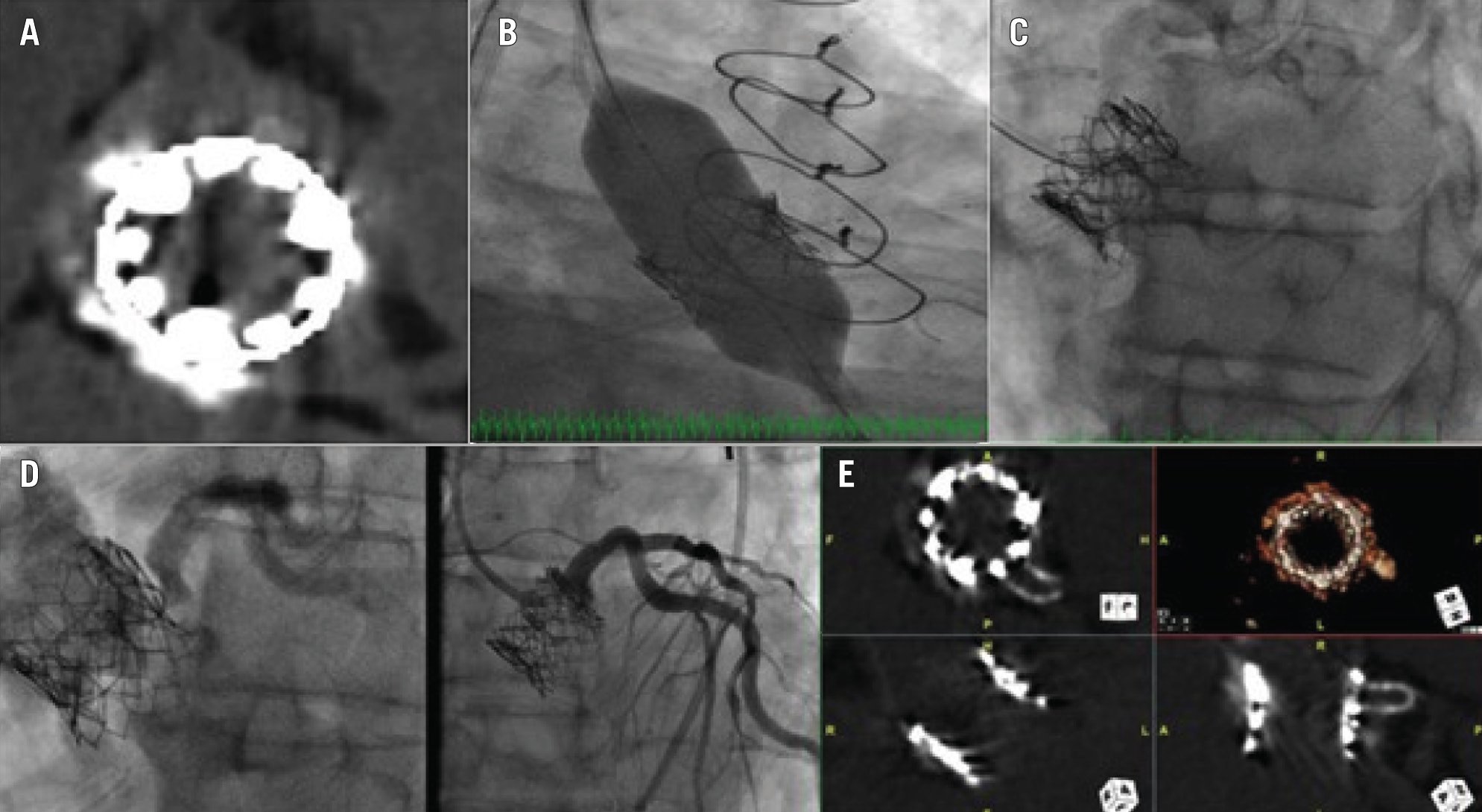
Figure 7. Treatment of a 79-year-old male who presented with critical aortic stenosis and had a history of CKD, SAVR and CABG (LIMA-LAD), redo-SAVR, PCI to LAD for LIMA graft failure, and valve-in-valve TAVI with a 23 mm SAPIEN XT for surgical bioprosthetic dysfunction. Coronary angiography showed patent stents. Non-contrast CT showed an LM height of 10 mm and a virtual transcatheter valve-to-coronary distance of 4 mm. The valve-to-aorta distance was 18 mm. Given the high likelihood of coronary obstruction, we preplanned the use of an electrosurgical bailout technique to recanalise the LM coronary artery. A) SAPIEN XT valve (Edwards Lifesciences) commissural alignment after valve-in-valve TAVI at 3 o’clock, 6 o’clock, and 10 o’clock. B) Transfemoral redo-TAVI was performed using a 23 mm SAPIEN S3 Ultra (Edwards Lifesciences). The LM was protected using a 6 Fr Amplatz Left 1 guide, guide extension catheter, coronary guidewire, and semicompliant coronary balloon in the LAD. C) Nearly 15 minutes after groin closure/haemostasis, the patient started complaining of severe chest pain with no ischaemic changes on ECG and normal left ventricular function on TTE. A non-selective angiogram demonstrated severe eccentric stenosis of the LM. A 7 Fr Judkins Left 4 guide was placed via the left femoral artery in the middle of the left sinus. An Astato XS 20 wire (Asahi Intecc) with a PiggyBack Wire Converter (Teleflex) was placed at the tip of the guide. Using electrosurgery at 70 W to perform a cut, the SAPIEN XT and surgical valve leaflets were punctured while injecting 5% dextrose. D) The patient was administered aspirin 325 mg and clopidogrel 600 mg orally. A 4×16 mm drug-eluting stent was proximally placed 1 mm outside the SAPIEN XT leaflet, as identified on intravascular ultrasound, at 14 atmospheres, and postdilatated with a 5.5×8 mm non-compliant balloon at 20 atmospheres. The final angiogram showed TIMI 3 flow in the LM and no residual stenosis. E) A non-contrast CT demonstrated fortuitous commissural alignment of the S3 Ultra posts with the SAPIEN XT posts. CABG: coronary artery bypass grafting; CKD: chronic kidney disease; CT: computed tomography; ECG: electrocardiogram; LAD: left anterior descending artery; LIMA: left internal mammary artery; LM: left main; PCI: percutaneous coronary intervention; SAVR: surgical aortic valve replacement; TAVI: transcatheter aortic valve implantation; TIMI: Thrombolysis in Myocardial Infarction; TTE: transthoracic echocardiogram
Treatment of coronary obstruction
In patients for whom coronary occlusion occurs without a protective guidewire in situ, immediate cannulation of the affected coronary artery with a guiding catheter is required to facilitate balloon angioplasty. In patients in whom coronary protection is performed, chimney stenting is used. The first left main (LM) occlusion post-TAVI at our institute was successfully stented after perforating the leaflet with the stiff end of the coronary guidewire82, though recently, with the advent of more complex electrosurgical techniques, we have found it feasible to lacerate the obstructing leaflet using a coronary wire with a high tip load and with application of the electrocautery pen. Coronary occlusion after selfâexpanding device deployment can be resolved by snaring the TAVI valve frame and lifting the deployed valve above the sinotubular junction. Haemodynamic support, including use of extracorporeal membrane oxygenation (ECMO), should be considered early to facilitate an interventional solution to the coronary obstruction. If, however, the situation cannot be resolved percutaneously, then emergent open-heart bypass remains the mainstay of treatment.
Valve malpositioning
Malpositioning of the valve during TAVI is exceedingly rare because of innovations in delivery systems that allow repositioning, recapture and retrieval. Self-expanding valves may be snared in the aortic direction if the valve is implanted too ventricular or aortic. Typically, the outer curvature is snared and the valve is pulled up. It can be secured in place with either a Palmaz stent (Cordis) or a BEV if necessary. In cases of balloon-expandable valve malpositioning, a second transcatheter valve is often necessary to stabilise the first. Special attention must be paid to not compromise coronary circulation from TAVI-in-TAVI as mentioned above. In cases of extreme ventricular deployment or ventricular valve embolisation, surgery to extract the valve prosthesis and perform aortic valve replacement may be required.
Acute kidney injury
The overall incidence of acute kidney injury (AKI) ranges from 10-40%, with stage 3 AKI observed in around 1% of patients undergoing TAVI. Encouragingly, the incidence of AKI in contemporary cohorts is down to around 10%, which may reflect the changes in the clinical profile of patients undergoing TAVI or may be related to improvements in procedural techniques83. However, numerous studies have consistently shown that the development of post-TAVI AKI is associated with adverse acute and longer-term morbidity, mortality, and quality of life. An analysis of >100,000 patients from the STS TAVI registry demonstrated an almost 7-fold increase in the hazard of 1-year mortality amongst patients who developed stage 3 AKI, compared to those who did not84. Baseline renal dysfunction is one of the strongest independent risk factors for the long-term mortality and development of post-TAVI AKI. In addition, comorbidities including anaemia, diabetes, chronic obstructive pulmonary disease, aortic or peripheral vascular disease are other risk factors. Severe renal artery calcification (RAC) has also been shown to be associated with lower odds of improvement in renal functions. During the TAVI procedure, the kidneys can be prone to injury either due to haemodynamic instability during rapid pacing, significant bleeding, and prosthesis deployment or due to embolism of atherosclerotic or calcific microfragments during catheter manipulation.
Prevention of AKI
For patients at high risk of AKI, adequate prehydration, particularly when combined with close monitoring of volume status – either conventionally or potentially with the use of modern techniques such as the RenalGuard system (CardioRenal Systems) – is an important consideration. For patients with baseline renal dysfunction, contrast-sparing strategies including the use of alternative imaging modalities, such as magnetic resonance imaging (MRI) or three-dimensional (3D) TOE, for preprocedural planning and intraprocedural guidance should be considered. We still recommend non-contrast-gated chest/abdomen/pelvis CT in these situations to better understand aortic root/leaflet calcification as well as access site assessment. Low-contrast volume CT protocols which provide adequate assessment of aortic and peripheral vessels have been described. Preprocedural TAVI CT can be performed with cardiac gating to evaluate coronary arteries, obviating the need for invasive angiography and thereby further reducing the total contrast volume administered preprocedurally. Alternatively, echocardiography, gadolinium-free cardiac magnetic resonance tomography, and fusion angiography can be used with procedural adaptions to perform an almost zero-contrast procedure. Other possible ways to mitigate the risk of AKI include angiography with diluted contrast, holding nephrotoxic drugs in the periprocedural phase, and substituting digital subtraction angiography with an ultrasound check at the end of procedure. Even in patients with significant CKD, meticulous attention should be given to vascular access assessment (including post-TAVI CFA angiography) to avoid vascular complications, especially major bleeding85.
Mital transcatheter edge-to-edge repair
The M-TEER procedure involves five steps: (1) large-bore venous access; (2) transseptal puncture; (3) device navigation in the left atrium; (4) leaflet grasping; and (5) device deployment. Below, we describe the complications associated with the use of M-TEER and their management (Table 3).
Table 3. Summary of complications after M-TEER and TMVR, prevention strategies and their subsequent treatment.
| Entête ajoutée | ||
|---|---|---|
| Complications following M-TEER | Prevention strategies | Treatment strategies |
| Transseptal puncture complications | Best position for transseptal puncture is in posterior and superior locations in the interatrial septumPerforming good transseptal puncture under TOE guidance | In case of pericardial effusion/tamponade, perform pericardial drainage and reversal of anticoagulation. There is a possibility of need for surgery. |
| Thrombus formation | Maintain activated clotting time between 250 and 300 seconds | If clot is seen during the procedure, it is best to remove the sheath and try to suction the clot as the sheath is being removed. |
| Single leaflet device attachment | Meticulous TOE assessment during leaflet grasping and device deploymentLive 3D multiplanar reconstruction | Additional clip or surgical management or conservative therapy |
| MitraClipa embolisation | Emergent mitral valve surgery | |
| Residual MR after MitraClip | Placing additional clipsTranscatheter occluder devicesELASTA-Clip | |
| Iatrogenic mitral stenosis after MitraClip | Appropriate preprocedural imaging, patient selection, and minimising the number of clip implantations by measuring mean mitral gradients after each clip implantation can help prevent iatrogenic mitral stenosis | |
| Complications following TMVR | Prevention strategies | Treatment strategies |
| LVOT obstruction | LAMPOONBATMANAlcohol septal ablationRadiofrequency ablationSESAME | ASA as bailout strategy |
| Valve embolisation/migration | Transcatheter snaring, redo transcatheter ViV, or open-heart surgery | |
| Cerebrovascular events | DOACs comparable with VKAs for thrombotic event prevention | |
| aby Abbott. 3D: three-dimensional; ASA: alcohol septal ablation; BATMAN: balloon-assisted translocation of the mitral anterior leaflet; DOAC: direct oral anticoagulant; ELASTA-Clip: electrosurgical laceration and stabilisation of failed MitraClip; LAMPOON: laceration of the anterior mitral leaflet to prevent outflow obstruction; LVOT: left ventricular outflow tract; MR: mitral regurgitation; M-TEER: mitral transcatheter-edge-to-edge repair; SESAME: septal scoring along the midline endocardium; TMVR: transcatheter mitral valve replacement; TOE: transoesophageal echocardiography; ViV: valve-in-valve; VKA: vitamin K antagonist | ||
Venous access complications
The procedure requires 24 Fr access through the femoral vein. Major bleeding requiring blood transfusion was seen in about 13.4% of patients in the Endovascular Valve EdgeâtoâEdge Repair Study (EVEREST) highârisk cohort86. Analysis from the TVT registry87 noted an incidence of 3.9% for major bleeding. Venous access complications can be prevented using ultrasound guidance for access and preclosure of the access site using a closure device or figure-of-eight sutures. Accidental arterial injury with a big sheath may require an arterial stent graft, but this is totally preventable by using ultrasound-guided entry.
Transseptal puncture complications
Transseptal puncture is usually performed in a posterior and superior location in the interatrial septum to allow sufficient height above the mitral annulus for device navigation. Inadvertent misdirected advancement of a transseptal needle can result in pericardial effusion and tamponade. In this scenario, percutaneous drainage of the effusion is performed, and the anticoagulation is reversed.
Thrombus formation
In patients with severe mitral regurgitation (MR), atrial fibrillation is common and coexistent mitral stenosis (MS) is not infrequently seen, either native valve MS or iatrogenic following M-TEER. These factors may contribute to thrombus formation. Cases have been reported of thrombus formation at the transseptal puncture site88. It is important to maintain an activated clotting time of between 250 and 300 seconds for the duration of the procedure. If a clot is seen during the procedure, it is best to remove the sheath and try to suction the clot as the sheath is being removed.
Single leaflet device attachment
Single leaflet device attachment (SLDA) is defined as the loss of a single device leaflet during insertion against the opposing leaflet. SLDA was seen in 2.2% of patients in the EVEREST highârisk registries86, 4.8% of patients in the ACCESS-EU registry89, and 1.5% in the TVT registry87. Risk factors for SLDA include flail leaflet, a large effective regurgitant orifice area (EROA), and a small mitral valve (MV) orifice area86. Options for managing SLDA include placement of an additional clip, surgical MV repair or medical management. Data from the ACCESS-EU registry90 showed that of the 27 patients who had SLDA (out of 567 included patients), 10 underwent a second MitraClip procedure, 6 underwent surgical MV repair or replacement, and 11 were treated medically. Most clips detach acutely (during the procedure) or subacutely (first days after the procedure), while late SLDA is infrequent. Meticulous TOE assessment during leaflet grasping and device deployment helps prevent SLDA. In our practice, contemporary TOE imaging using live 3D multiplanar reconstruction is the default modality and has proven invaluable in both preprocedural diagnostic imaging and procedural guidance (Figure 8, Supplementary Figure 1). In addition to the live images, acquisition of full 3D volumes allows the imaging specialist to pause, reanalyse and reconstruct the grasping phases in an almost infinite number of planes to provide either reassurance of clip stability before release or demonstrate the need for regrasping. Appropriate leaflet grasping should be confirmed in multiple TOE views, including the longâaxis view prior to device deployment. Kreidel et al90 reported their experience from Germany of 21 patients undergoing a second MitraClip procedure for recurrent MR. They observed an 85% success rate of repeated MitraClip in patients without loss of leaflet insertion (LLI) versus only 25% success in those with LLI. In a multicentre registry91, a total of 147 cases of MitraClip failure were noted; these were secondary to LLI in 31.9% of cases, SLDA in 67.3% of cases, and clip embolisation in 1 case. In all, 48% had conservative management, followed by MitraClip in 35%, and surgery in 17%. Survival analysis suggested a trend towards a better outcome for those patients with redo MitraClip, and multivariable analysis demonstrated a survival benefit for redo MitraClip.
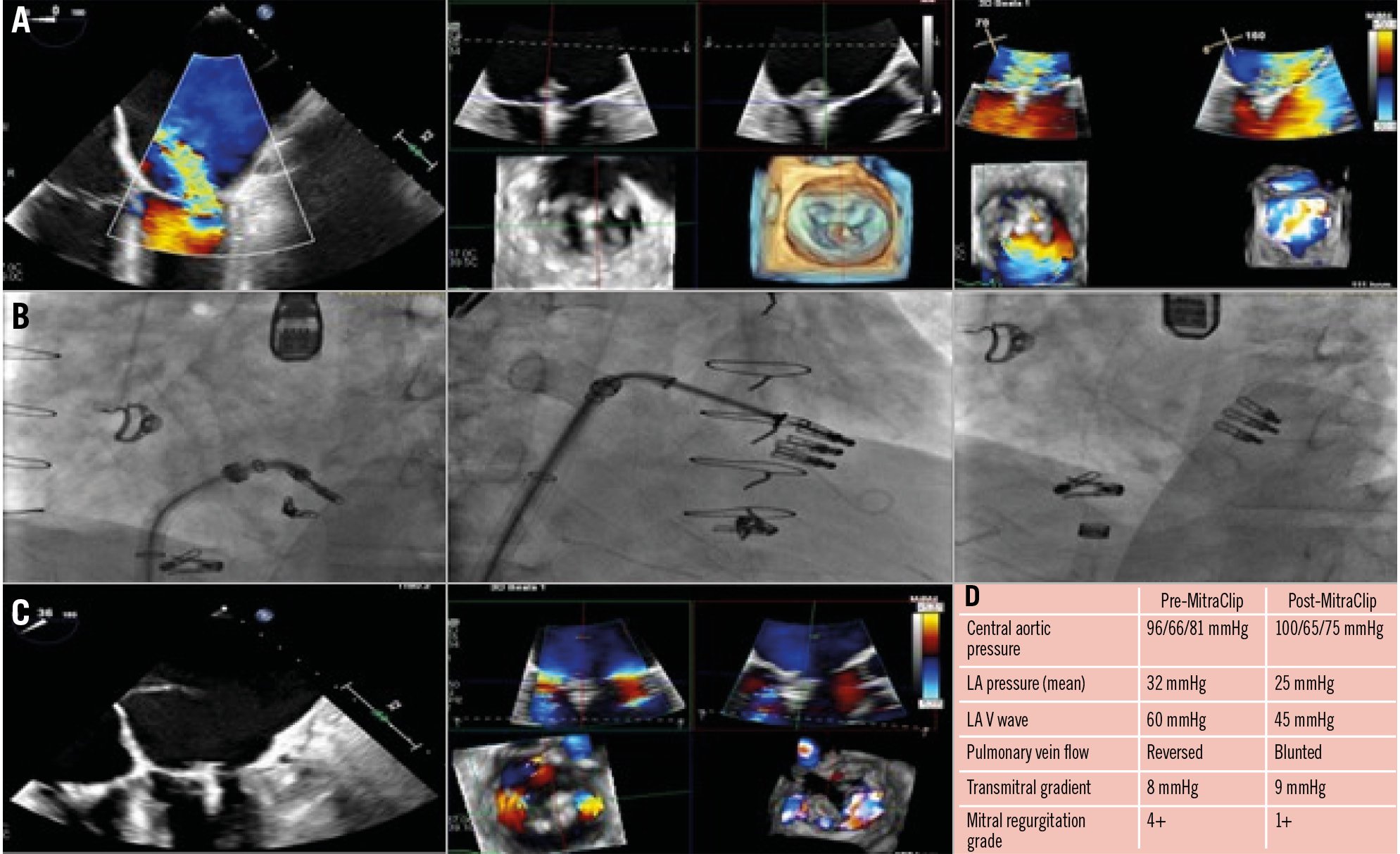
Figure 8. Treatment of a 52-year-old male with a history of dilated cardiomyopathy s/p heart transplant, MR and TR s/p mitral and tricuspid TEER (1 MitraClip XT [Abbott] on the MV, 2 XTs on the TV), and CKD, with recurrent severe MR in the setting of single leaflet detachment. A) TOE images showing severe MR (4+) with anterior translocation of the posterior clip with attachment to posterior leaflet tips (SLDA) along with moderate-severe TR (3+) originating adjacent to the clips. B) Fluoroscopy images. First, the MitraClip XT was placed medial to the prior clip, and then a second MitraClip XT was placed lateral to the prior clip. C) TOE images with a reduction in MR grade from 4+ to 1+ following redo MitraClip. There was a significant improvement in haemodynamics, as shown (D). CKD: chronic kidney disease; LA: left atrial; MR: mitral regurgitation; MV: mitral valve; SLDA: single leaflet device attachment; s/p: status/post; TEER: transcatheter edge-to-edge repair; TOE: transoesophageal echocardiogram; TR: tricuspid regurgitation; TV: tricuspid valve
Dislocation of existing pacemaker lead
To avoid this complication, it is important to check, with fluoroscopy and echocardiography, the relation between the transseptal puncture catheter and the leads during manoeuvring through the right atrium88.
Residual MR after the MitraClip
It is well established that ≥2+ residual MR at discharge has significantly lower survival rates compared to less MR92. In most cases, it can be treated by placing additional clips if the transmitral gradients and anatomy allow it. However, placing additional clips can sometimes be technically challenging because of the complex MV anatomy. Transcatheter occluder devices have been used as a bailout strategy in cases where the placement of additional clips is not feasible, either between clips or between a clip and the commissure. Other novel techniques like electrosurgical laceration and stabilisation of failed MitraClip (ELASTA-Clip) have been described to facilitate TMVR for significant residual MR after MitraClip93.
Iatrogenic mitral stenosis after MitraClip
Predictors of elevated mitral valve gradients postâMitraClip include preprocedural MV area <4.0 cm2, more than one clip implantation, and elevated baseline mean MV gradients. A mean diastolic gradient of >5 mmHg across the MV after MitraClip implantation is associated with worse outcomes, including allâcause mortality. Appropriate preprocedural imaging, patient selection, and minimising the number of clip implantations by measuring mean MV gradients and MR grades after each clip implantation can help prevent iatrogenic MS. A balance between the degree of MR reduction and MV gradients should be maintained throughout the procedure. If the mean gradient across the MV is >7 mmHg and the MV area is <3 cm2, additional MitraClips should not be placed. Not tightening the clip fully has been tried but cannot be routinely recommended. One theoretical consideration is to use an M-TEER device with a spacer such as PASCAL (Edwards Lifesciences), but in practice, the gradients are not lower compared to using a clip in randomised trials.
Iatrogenic atrial septal defect after MitraClip
With an increase in the utilisation of MitraClip, the consequences of transseptal puncture resulting in iatrogenic atrial septal defects (iASDs) are being recognised. The prevalence of persistent iASDs 1 month post-MitraClip range between 43% and 82%. The higher prevalence of persistent iASDs after MitraClip procedures is attributed to larger catheter sizes, longer procedure durations, extensive sheath movement, and elevated left atrial pressures. Closure for iASDs is recommended in patients at high risk, including large iASDs (over 8 mm), large left-to-right shunt, right-to-left shunt with evidence of hypoxaemia, severe right ventricular dysfunction, risk of paradoxical embolus, aneurysmal septum, and pacemaker leads. In a randomised trial setting, iASD closure for persistent iASDs 1 month after a MitraClip procedure has not been shown to improve clinical outcomes94.
Transcatheter mitral valve replacement
Below, we have summarised the major complications following transcatheter mitral valve replacement and their management (Table 3). Other complications including valve embolisation, LV perforation, cerebrovascular events, and paravalvular leak after TMVR have been described in Supplementary Appendix 195969798.
Left ventricular outflow tract obstruction
LVOT obstruction (LVOTO) is a predictor of poor outcome after TMVR and is a common reason for exclusion from TMVR therapy99. Risk of LVOTO can be accurately predicted by gated cardiac CT analysis. Factors that are taken into consideration when analysing preprocedural imaging are the anterior mitral leaflet length, neo-LVOT area <200 mm2, device-related dimensions, aortomitral angle, and basal septal bulge100. In an analysis of approximately 200 cases from the TMVR international multicentre registry, the prevalence of LVOTO was 13%, with the highest rate in valve-in-mitral annular calcification (MAC), then in valve-in-ring, and ViV procedures (54%, 8%, and 2%, respectively)101. Several novel therapeutic strategies have shown efficacy at reducing the risk of LVOTO to facilitate TMVR. LVOTO can occur either because of the deflection of the anterior mitral leaflet towards the septum or because of excess basal septal bulge. The former can be treated with laceration of the anterior mitral leaflet to prevent outflow obstruction (LAMPOON). In the initial experience of the LAMPOON procedure102, 15 patients with severe MAC and 15 patients with prior annuloplasty underwent LAMPOON-facilitated TMVR. All patients underwent successful laceration of the anterior leaflet and TMVR, although 10% of patients still experienced significant LVOTO despite anterior leaflet laceration. Thirty-day survival was 93%. Other novel techniques studied include balloon-assisted translocation of the mitral anterior leaflet (BATMAN)103. In cases of excess septal bulge, alcohol septal ablation (ASA) can be utilised to prevent LVOTO. In the studies, ASA has been utilised as a bailout strategy as well as a precautionary measure in high-risk patients104. Other solutions in the case of excess basal septal hypertrophy include radiofrequency ablation and septal scoring along the midline endocardium (SESAME)105. There have also been reports of transapical mitral leaflet cutting to prevent LVOTO99106. In Supplementary Figure 2, we have presented a case of concomitant redo-TAVI and TMVR in MAC where PVL was also plugged. TAVI is generally performed first to prevent the displacement of the MV during TAVI deployment or post-dilatation.
Afterload mismatch post-TMVR
Afterload mismatch post-TMVR refers to acute impairment of left ventricular function that occurs when there is an increase in afterload following correction of MR. It requires careful management in the acute setting to support the left ventricle and ensure patient stability. Management may include pharmacological support with inotropes or vasodilators to reduce afterload and optimise left ventricular function. Monitoring and managing right ventricular function and pulmonary hypertension are also important, as these can be exacerbated by afterload mismatch.
Conclusions
In summary, as transcatheter valve interventions expand to include lower-risk patient populations, it becomes imperative to take the utmost measures to prevent complications. This fundamental need continues to drive the ongoing innovation of novel technologies for the prevention and treatment of complications following transcatheter valvular interventions.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

