
Abstract
Aims: We sought to investigate the influence of the target vessel on the prognostic relevance of fractional flow reserve (FFR) after percutaneous coronary intervention (PCI).
Methods and results: A total of 835 patients with available FFR after second-generation drug-eluting stent (DES) implantation were included in this study. The primary outcome was target-vessel failure (TVF), including cardiac death, target vessel-related myocardial infarction, and clinically driven target vessel revascularisation. The target vessel was the left anterior descending artery (LAD) in 603 patients (72.2%) and non-LAD in 232 patients (27.8%). The distribution pattern of post-PCI FFR values was different between the LAD and non-LAD (p<0.001). The optimal cut-off values of post-PCI FFR for predicting TVF were 0.82 and 0.88 in the LAD and non-LAD, respectively. The cumulative incidence of TVF was significantly higher in patients with lower post-PCI FFR than each cut-off value (10.9% vs. 2.5%, hazard ratio [HR] 4.08, 95% confidence interval [CI]: 2.63-6.34, p<0.001 in LAD; 8.0% vs. 1.9%, HR 6.00, 95% CI: 1.78-20.26, p=0.004 in non-LAD).
Conclusions: Higher post-PCI FFR after second-generation DES implantation was associated with better clinical outcomes. Different cut-off values of post-PCI FFR need to be applied according to the target vessel. ClinicalTrials Identifier: NCT01873560
Introduction
The presence of myocardial ischaemia is a key prognostic factor in patients with coronary artery disease. Coronary revascularisation is beneficial only for those with myocardial ischaemia1. Fractional flow reserve (FFR) is a standard invasive physiologic index used to define ischaemia-causing coronary artery stenosis2,3. In addition to pre-percutaneous coronary intervention (PCI) assessment, previous studies have demonstrated that FFR measured after stent implantation had prognostic implications4,5,6,7,8,9,10,11,12. However, optimal cut-off values of post-PCI FFR ranged widely, from 0.86 to 0.96.
FFR is influenced not only by stenosis severity but also by the amount of perfused myocardium. Therefore, the pressure gradient can be variable according to the supplying myocardium, even in vessels with the same stenosis severity13. As the amount of myocardium is significantly larger in the left anterior descending artery (LAD) territory than in the non-LAD territory, the clinical meaning of the same post-PCI FFR value between them can be different. However, the prognostic relevance of post-PCI FFR according to vessel location has not been thoroughly evaluated. We sought to investigate the prognostic relevance of post-PCI FFR and its optimal cut-off value according to the target vessel.
Methods
STUDY POPULATION
This study is based on an international, multicentre, prospective registry to evaluate the prognostic relevance of post-PCI FFR from nine hospitals in Japan and the Republic of Korea. A total of 939 consecutive patients who underwent PCI and post-PCI FFR measurements after angiographically successful stent implantation (residual stenosis <20% by visual estimation) between May 2013 and December 2016 were included in this registry. Exclusion criteria were post-PCI TIMI flow of <3, depressed left ventricular systolic function (ejection fraction <30%), culprit lesion for acute coronary syndrome, graft vessel, collateral feeder, in-stent stenosis, primary myocardial or valvular heart disease, and patients with life expectancy <2 years. Among enrolled patients, 29 had poor FFR data, four patients withdrew from the study, four were lost to follow-up and 67 were treated with bare metal stent or first-generation drug-eluting stent (DES), and were therefore excluded from the current analysis. Finally, a total of 835 patients were included in this study. Among the total study population, 11.4% of patients (95/835) underwent multivessel PCI. In these patients, the target vessel was defined as the vessel with the lowest post-PCI FFR value for the analysis. The study protocol was approved by the ethics committee at each participating centre and was conducted according to the principles of the Declaration of Helsinki (ClinicalTrials.gov Identifier: NCT01873560). All patients provided written informed consent.
INVASIVE CORONARY ANGIOGRAPHY, PCI AND MEASUREMENT OF FFR
Invasive coronary angiography was performed according to standard techniques. During PCI, type of DES, stenting techniques, and the use of additional imaging devices, such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT), were at the operator’s discretion.
All angiograms were analysed at a core laboratory (Inje University Ilsan Paik Hospital, Koyang, Republic of Korea) in a blinded fashion. With optimal projections, quantitative coronary angiography (QCA) was performed using a validated software programme (CAAS II; Pie Medical Imaging, Maastricht, the Netherlands).
FFR was measured using a 5-7 Fr guide catheter and a pressure sensor guidewire (St. Jude Medical, St. Paul, MN, USA, or Volcano, San Diego, CA, USA), as previously described14. To induce hyperaemia, a continuous infusion of adenosine (140 µg/kg/min) was used in most patients. Intracoronary administration of papaverine, nicorandil or adenosine was used in 189 patients.
DATA COLLECTION, FOLLOW-UP OF PATIENTS, AND STUDY ENDPOINTS
Patient demographic data, cardiovascular risk factors and clinical diagnoses were recorded at the time of index PCI. Clinical follow-up was performed at 1, 6, 12 and 24 months by outpatient clinical visits or telephone contact. The median follow-up duration of the study population was 710 (391-744) days. An external clinical events committee reviewed and adjudicated all relevant medical records for any clinical events.
The primary outcome was target vessel failure (TVF), defined as a composite of cardiac death, target vessel-related myocardial infarction (MI) and clinically driven target vessel revascularisation (TVR) up to two years. All deaths were considered cardiac unless an undisputed non-cardiac cause was present. Periprocedural MI was not counted for clinical outcome. Clinically driven revascularisation was defined as repeat revascularisation in the presence of a diameter stenosis ≥50% with at least one of the following: 1) recurrence of anginal symptoms; 2) positive non-invasive test; 3) positive invasive physiologic test; or 4) presence of diameter stenosis ≥70%, even in the absence of other criteria.
STATISTICAL ANALYSIS
Categorical variables are presented as numbers and relative frequencies. Continuous variables are presented as means and standard deviations or medians with interquartile ranges (IQR) according to their distribution. The Student’s t-test and Wilcoxon signed-rank test were performed for comparison of continuous variables according to the distribution of variables. The Kolmogorov-Smirnov test was used to compare the distribution pattern of post-PCI FFR values of the LAD and non-LAD. Linear by linear associations of categorical variables were evaluated using the chi-square test for trends. The optimal cut-off values of post-PCI FFR for predicting clinical outcomes were calculated based on maximising the sum of sensitivity and specificity of each index, using receiver operating characteristic (ROC) curve analysis.
Kaplan-Meier analysis was performed to calculate the cumulative incidence of clinical outcomes and the log-rank test for comparison of group differences. Univariate and multivariate adjusted Cox proportional hazard regression models, stratified by each included centre, were used to calculate the hazard ratio (HR) and 95% confidence interval (CI). The following patient characteristics were included in a multivariate adjusted Cox proportional hazards regression model: age, sex, hypertension, diabetes mellitus, hypercholesterolaemia, chronic kidney disease, previous MI, current smoking, left ventricular dysfunction and clinical diagnosis. The multivariate Cox proportional hazards regression analysis stratified by each included centre was performed to identify the independent predictors of TVF. Covariates that were considered clinically reliable or were associated with TVF (univariate analysis, p-value <0.1) were included in the model. All p-values were two-sided, and p<0.05 was considered statistically significant. The statistical package R, version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis.
Results
PATIENT AND LESION CHARACTERISTICS
Baseline characteristics are presented in Table 1. Among 835 patients, 651 patients (78.0%) were male and the median age was 64.0 (56.0-72.0) years. A total of 135 patients (16.2%) demonstrated a post-PCI FFR <0.80. Post-PCI FFR values were not significantly different between patients with acute coronary syndrome and those with stable angina (0.85 [0.82-0.90] vs 0.85 [0.81-0.88], p=0.061 in LAD; 0.92 [0.86-0.97] vs 0.91 [0.85-0.96], p=0.298 in non-LAD). Coronary imaging, such as IVUS or OCT, was used in 479 (57.4%) patients for stent optimisation. During post-PCI FFR measurement, four cases (0.5%) of complications occurred (three cases of coronary spasm, and one case of coronary thrombosis).
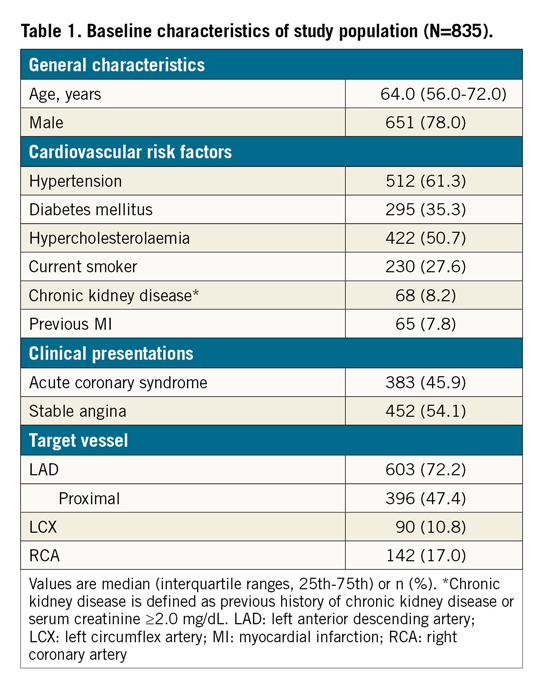
The target vessel was the LAD in 603 patients (72.2%). Although the angiographic stenosis was more severe in the non-LAD group than in the LAD group (diameter stenosis 70.0% [63.0-77.1] vs 64.0% [57.0-73.0], p<0.001), baseline FFR was higher in the non-LAD group (0.72 [0.63-0.78] in non-LAD vs 0.70 [0.62-0.76] in LAD, p=0.018) (Table 2). After stent implantation, there was no difference in angiographic residual stenosis between the two groups. However, post-PCI FFR was higher in the non-LAD group than in the LAD group (0.92 [0.85-0.96] vs 0.85 [0.81-0.89], p<0.001) (Table 2). The distribution pattern of post-PCI FFR values was significantly different between the LAD and non-LAD groups (p<0.001) (Figure 1, Table 2).
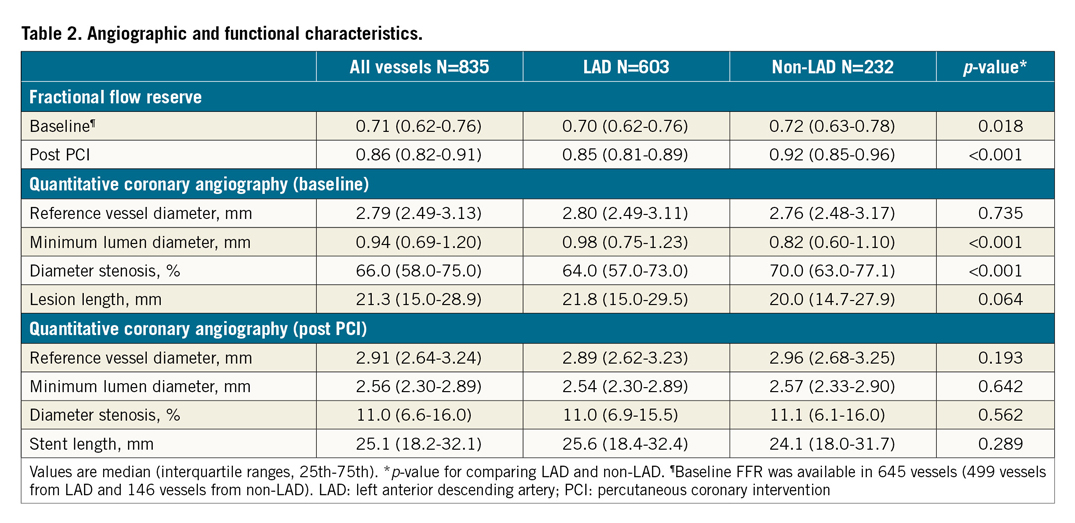
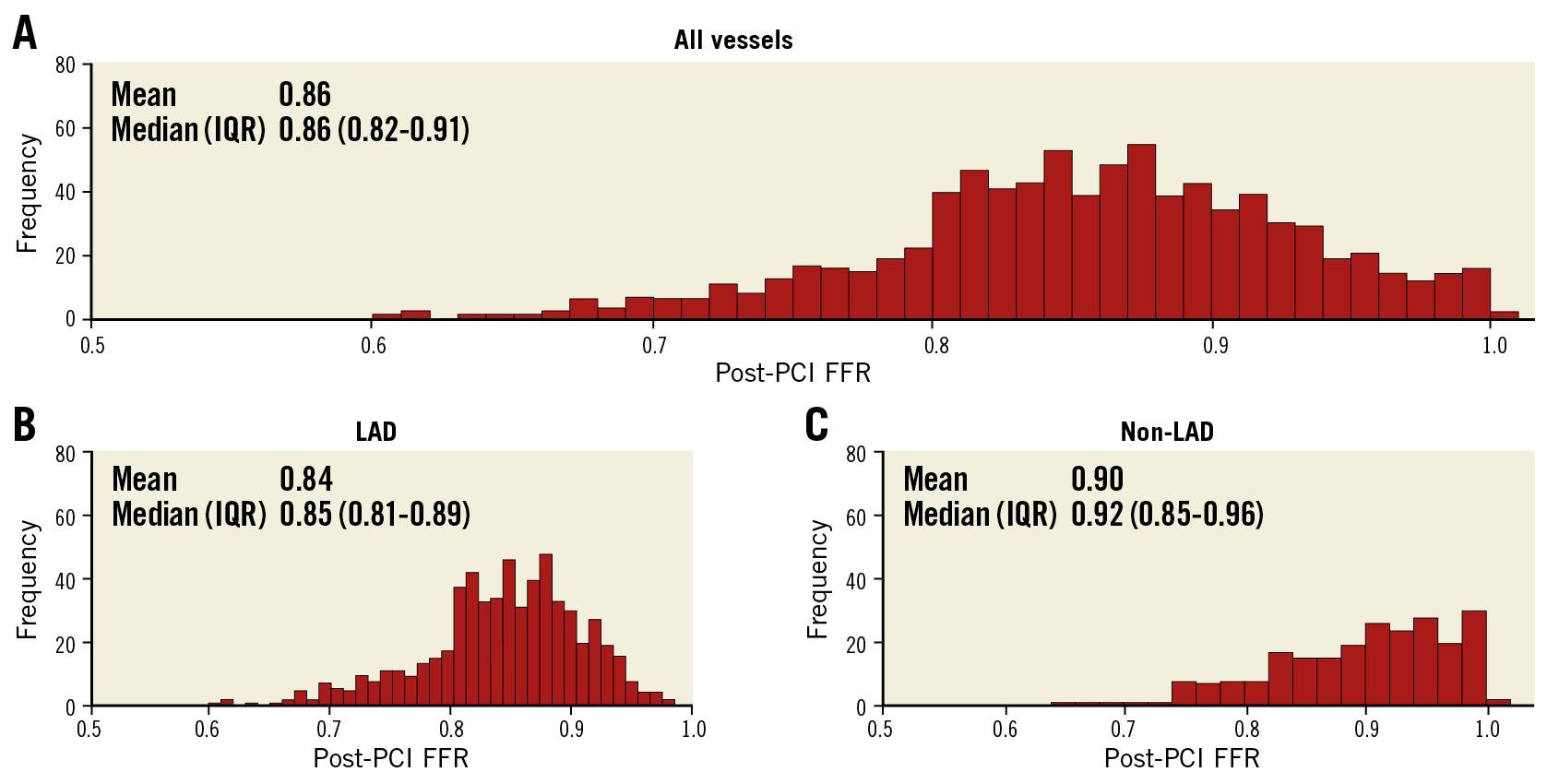
Figure 1. Distributions of post-PCI FFR. Distributions of post-PCI FFR in all vessels (A), LAD (B) and non-LAD (C) are shown. FFR: fractional flow reserve; IQR: interquartile range; LAD: left anterior descending artery; PCI: percutaneous coronary intervention
CLINICAL OUTCOMES ACCORDING TO BASELINE AND POST-PCI FFR
There was no association between baseline FFR values and the rates of TVF (p for trend=0.978) (Figure 2A). On the contrary, post-PCI FFR demonstrated a significant association with TVF. The rates of TVF were increased along with a decrease of post-PCI FFR (p for trend <0.001; adjusted HR 1.08 per 0.01, 95% CI: 1.06-1.11, p<0.001) (Figure 2B). Among 10 TVRs in patients with post-PCI FFR below 0.80, four cases occurred in stented segments.
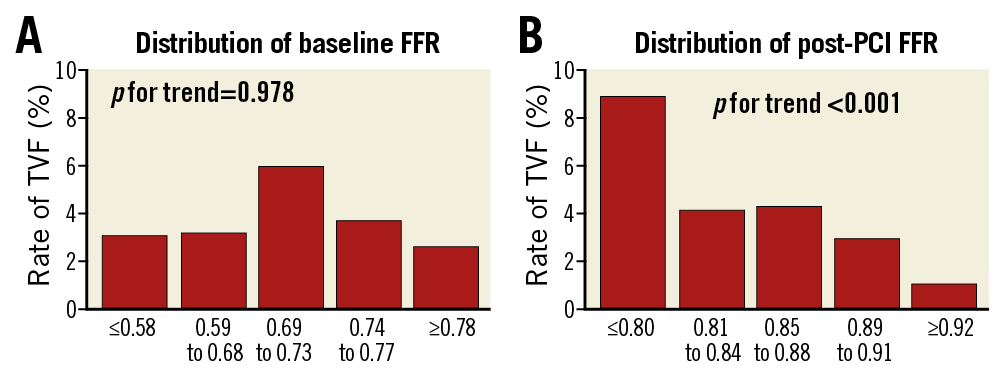
Figure 2. Clinical outcomes according to baseline and post-PCI FFR. The rates of TVF are shown according to quintiles of baseline FFR (A) and post-PCI FFR (B). FFR: fractional flow reserve; PCI: percutaneous coronary intervention; TVF: target vessel failure
OPTIMAL CUT-OFF VALUE OF POST-PCI FFR
The optimal cut-off values of post-PCI FFR for predicting TVF are presented in Figure 3. In all vessels, the optimal cut-off value of post-PCI FFR was 0.84, and sensitivity, specificity and area under the curve (AUC) were 65.1%, 61.3% and 0.696, respectively. The optimal cut-off values of post-PCI FFR in the LAD and non-LAD were different: 0.82 (sensitivity 67.6%, specificity 61.2%, AUC 0.704) and 0.88 (sensitivity 66.1%, specificity 64.5%, AUC 0.721), respectively. The rates of achieving optimal cut-off values according to the target vessel were 65.5% and 64.2% in the LAD and non-LAD, respectively. The optimal cut-off values of post-PCI FFR for predicting TVF were not changed after excluding multivessel PCI.
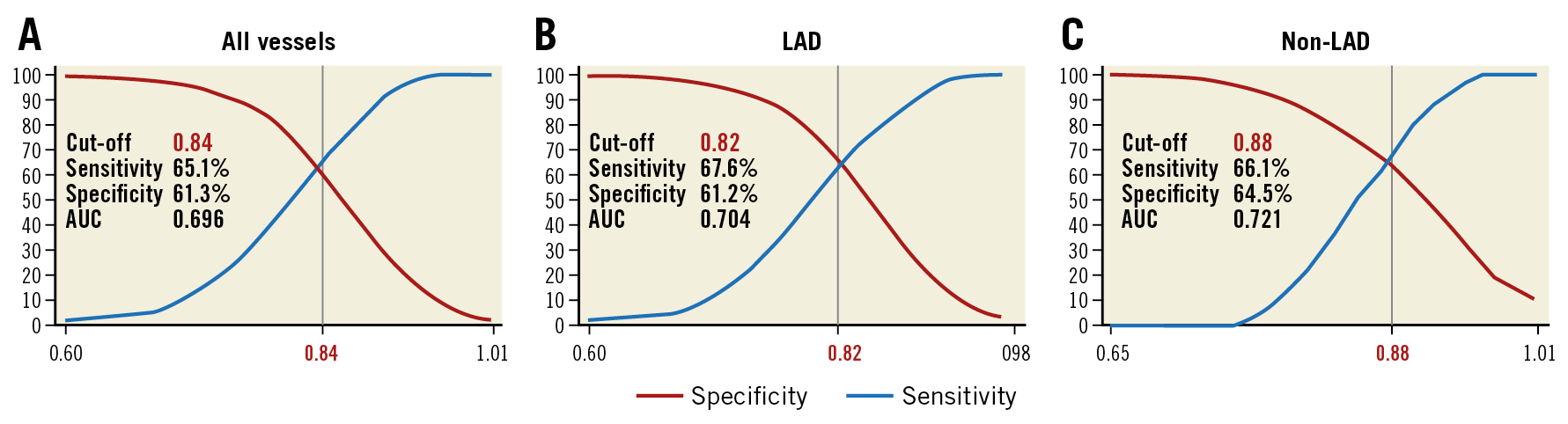
Figure 3. Optimal cut-off values of post-PCI FFR. Receiver operating characteristic curve analysis was performed to calculate optimal cut-off values of post-PCI FFR in all vessels (A), LAD (B) and non-LAD (C). AUC: area under the curve; FFR: fractional flow reserve; LAD: left anterior descending artery; PCI: percutaneous coronary intervention
CLINICAL OUTCOMES AND POST-PCI FFR ACCORDING TO THE TARGET VESSEL
With the optimal cut-off value in all vessels, patients with post-PCI FFR ≤0.84 demonstrated significantly higher cumulative incidence of TVF than patients with post-PCI FFR >0.84 (8.3% vs 3.1%; HR 2.51, 95% CI: 1.51-4.16, p<0.001; adjusted HR 2.98, 95% CI: 1.92-4.61, p<0.001) (Table 3). However, when the cut-off value of 0.84 was applied to the non-LAD lesions, there was no statistically significant difference in outcomes between the high and low post-PCI FFR groups (HR 2.30, 95% CI: 0.46-11.53, p=0.311; adjusted HR 3.35, 95% CI: 0.62-18.08, p=0.161) (Supplementary Table 1).
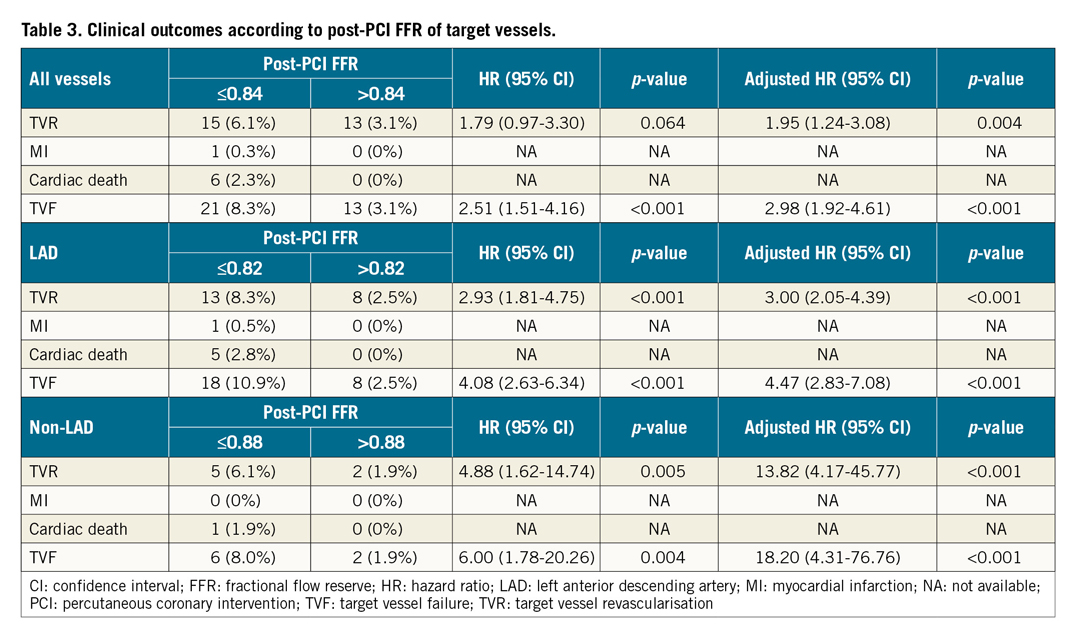
When the different cut-off values were used according to the target vessel (LAD 0.82, non-LAD 0.88), the cumulative incidence of TVF was significantly higher in patients with lower post-PCI FFR than each cut-off value (10.9% vs 2.5%, HR 4.08, 95% CI: 2.63-6.34, p<0.001 in LAD; 8.0% vs 1.9%, HR 6.00, 95% CI: 1.78-20.26, p=0.004 in non-LAD) (Figure 4, Table 3). In multivariate analyses, the results were the same as in the unadjusted analyses. These results were not changed after excluding those with multivessel PCI from the analysis (Supplementary Table 2).
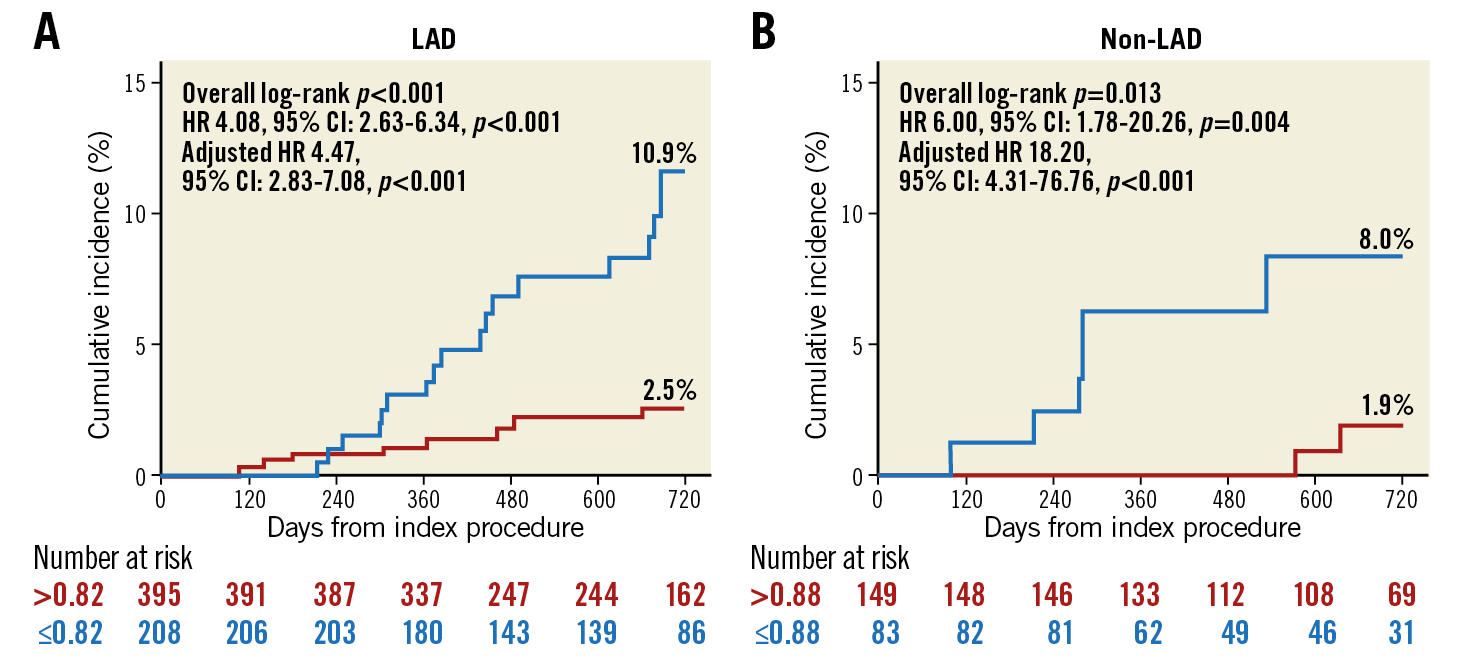
Figure 4. Cumulative incidence of TVF according to optimal cut-off values of post-PCI FFR. Kaplan-Meier curves of TVF according to the optimal cut-off values of the target vessels are presented. The optimal cut-off values of post-PCI FFR for predicting TVF were 0.82 in the LAD (A) and 0.88 in the non-LAD (B) vessels, respectively. CI: confidence interval; FFR: fractional flow reserve; HR: hazard ratio; LAD: left anterior descending artery; PCI: percutaneous coronary intervention; TVF: target vessel failure
The lower post-PCI FFR than each cut-off from the LAD and non-LAD was an independent predictor of TVF (adjusted HR 6.80, 95% CI: 3.33-13.86, p<0.001) (Supplementary Table 3). Neither baseline FFR nor % diameter stenosis before and after PCI was associated with the occurrence of TVF.
Discussion
The current study evaluated the clinical relevance of post-PCI FFR after second-generation DES implantation as well as the influence of the target vessel on the clinical value of post-PCI FFR. The principal findings of this study were as follows. First, both baseline and post-PCI FFR values were higher in the non-LAD group than in the LAD group, and the distribution pattern of post-PCI FFR was different in the LAD and non-LAD. Second, although baseline FFR was not associated with TVF, the rates of TVF increased along with a decrease of post-PCI FFR. Third, optimal cut-off values of post-PCI FFR were different according to the target vessel and were 0.82 and 0.88 in the LAD and non-LAD, respectively. Finally, each cut-off value of post-PCI FFR according to the target vessel was associated with the risk of TVF, and low post-PCI FFR was an independent predictor of TVF.
CURRENT EVIDENCE ON POST-PCI FFR
FFR has been a standard invasive physiologic index used to define the presence of myocardial ischaemia2,3. In addition to FFR’s role in pre-PCI treatment decision making, previous studies have also reported the prognostic implications of post-PCI FFR4,5,6,7,8,9,10,11,12. Since Pijls et al reported that post-PCI FFR after bare metal stent implantation was an independent predictor of clinical events at six months4, many studies, including meta-analyses, have shown that higher post-PCI FFR is associated with better clinical outcomes4,5,6,7,8,9,10,11,12. However, the optimal cut-off value of post-PCI FFR is still controversial, and the proposed values ranged widely, from 0.86 to 0.964,5,6,7,8,9,10,11,12. This might result from differences in study populations, definition of outcomes, type of stent used, and distribution of included vessels among previous studies.
TARGET VESSEL AND POST-PCI FFR
FFR is a pressure-derived physiologic index influenced not only by lesion severity but also by the amount of coronary flow15. Therefore, coronary flow and pressure gradient across the lesion can be greater when the diseased vessel supplies larger myocardium even with the same stenosis severity. In our study, baseline FFR values were lower in the LAD group than in the non-LAD group, despite lower angiographic % diameter stenosis. FFR was still lower in the LAD group than in the non-LAD group after stent implantation with similar residual stenosis. As myocardial mass and coronary flow are greater in the LAD than in the non-LAD, FFR value can be inherently lower in the LAD than in the non-LAD (Figure 5). These results are in line with previous studies which reported that a LAD lesion was an independent predictor of low FFR value9,10,13,16. In addition, the distribution pattern of post-PCI FFR was different between the LAD and non-LAD in our study. These results suggest that the clinical meaning of the same post-PCI FFR values between the LAD and non-LAD can be different. This may be one of the reasons for various cut-off values of post-PCI FFR in previous studies4,5,6,7,8,9,10,11,12. The current study showed that the optimal cut-off values for predicting TVF could be different in the LAD and in the non-LAD vessels.
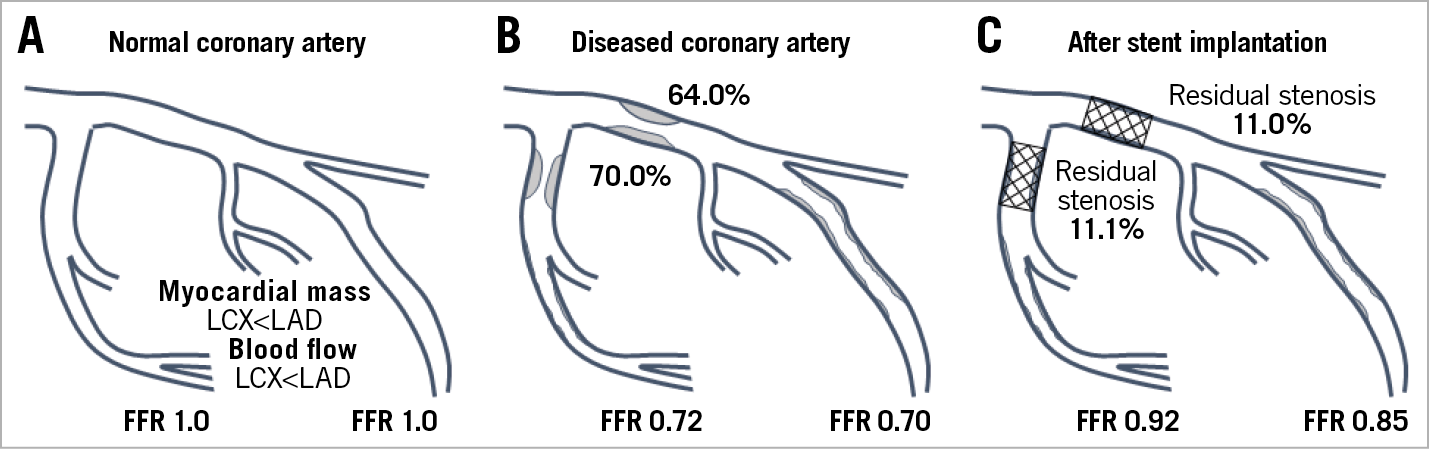
Figure 5. Stenosis severity and FFR in normal, diseased, and stented vessels. Myocardial mass and coronary blood flow are larger in the LAD territory than in the non-LAD territory (A). Stenosis severity by angiographic diameter stenosis and FFR values from this study, before (B) and after (C) coronary stenting, are presented. FFR: fractional flow reserve; LAD: left anterior descending artery; LCX: left circumflex artery
CLINICAL RELEVANCE OF POST-PCI FFR ACCORDING TO THE TARGET VESSEL
This study focused on the clinical value of post-PCI FFR according to the target vessel. In our study, the optimal cut-off value of post-PCI FFR derived from all vessels (0.84) could not discriminate the risk of TVF in non-LAD vessels. With different distribution patterns of FFR values according to the target vessel, these results showed the limitations of applying one cut-off value of post-PCI FFR in predicting clinical outcomes after coronary stenting. Recently, Li et al reported the optimal cut-off value of post-PCI FFR in all vessels (0.88) as well as that in the LAD (0.905)9. While this report did not describe post-PCI FFR and clinical outcomes according to the target vessel in detail, the optimal cut-off value of post-PCI FFR in all vessels was achieved less frequently in the LAD than in either the left circumflex or right coronary arteries (59.0% vs 85.7%), as in our study. The difference in optimal cut-off values of post-PCI FFR between our study and Li et al’s could be because of the difference in study population, lesion and procedural characteristics, and the incidence of clinical events. As the optimal cut-off value of post-PCI FFR is affected by various factors, no single study is able to define the conclusive cut-off value of post-PCI FFR. Taken together, both studies suggest the clinical relevance of applying different post-PCI FFR cut-off values based on different target vessels.
While focusing on the clinical implications of post-PCI FFR according to the target vessel, this study could not explain the influence of interaction between stented and non-stented segments on FFR values and clinical outcomes. However, it is important to understand that there can be complex interactions between stented and non-stented segments for determination of post-PCI FFR and clinical outcomes. A more sophisticated study using pre- and post-PCI FFR and pre- and post-PCI invasive imaging studies in a large number of patients is needed to investigate this relationship clearly. In this context, instantaneous wave-free ratio pullback can be helpful to understand the physiologic contribution of each segment before and after coronary stenting17.
Limitations
There were several limitations in this study. First, the post-PCI FFR values were not blinded to the physicians. This could be a source of bias as TVR was the main contributor of clinical events. Second, there are inherent limitations of an observational registry study. Third, pullback recordings of post-PCI FFR and intravascular imaging were not mandatory in this study. Therefore, the reasons for suboptimal post-PCI FFR values could not be assessed in all patients. However, as the post-PCI FFR used in this study was measured after clinically and angiographically successful PCI, this study’s results provide information on the distribution and clinical relevance of post-PCI FFR in daily practice. Fourth, this study could not provide conclusive optimal cut-off values of post-PCI FFR according to the target vessel due to the relatively small number of patients and events included. Further study is needed to explore the conclusive optimal cut-off values of post-PCI FFR according to the target vessel. Last, the presence of microvascular dysfunction was not systematically assessed. Therefore, this study could not explain the potential influence of microvascular disease on post-PCI FFR.
Conclusions
Higher post-PCI FFR was associated with better clinical outcomes after second-generation DES implantation. Different cut-off values of post-PCI FFR may need to be applied according to the target vessel to optimise clinical outcomes further for patients with coronary artery disease. However, it needs to be emphasised that post-PCI FFR is one of the components that can determine the clinical outcomes in patients with coronary artery disease after stent implantation.
|
Impact on daily practice With different distribution patterns of post-PCI FFR values according to the target vessel, optimal cut-off values of post-PCI FFR were different according to the target vessel and were 0.82 and 0.88 in the left anterior descending coronary artery (LAD) and non-LAD, respectively. Each cut-off value of post-PCI FFR according to the target vessel was strongly associated with clinical outcomes. This study suggests the clinical relevance of applying different post-PCI FFR cut-off values based on different target vessels. |
Acknowledgements
J-H. Doh and B-K. Koo contributed equally to the study design, data collection and study supervision for this study.
Funding
This work was supported by a research grant (No. 201002-01) from the Korean Circulation Society.
Conflict of interest statement
B-K. Koo and J.M. Lee have received an institutional research grant from St. Jude Medical (Abbott) and Philips Volcano. J-H. Doh has received an institutional research grant from Philips Volcano. J-Y. Hahn has received an institutional research grant from St. Jude Medical (Abbott) and Boston Scientific. The other authors have no conflicts of interest to declare.

