Abstract
Aims: Fractional flow reserve (FFR) ≥0.96 after stenting correlates well with an optimal stent expansion, but outcomes based on FFR after drug eluting stents (DES) have not been studied. This study sought to investigate the proportion of patients in whom an FFR ≥0.96 can be achieved after transradial stenting using primarily DES and to determine outcomes based on a post-stent FFR ≥0.96 vs. an FFR<0.96.
Methods and results: A total of 66 patients with single-vessel disease and FFR<0.75 underwent transradial stenting. After stenting, FFR was <0.96 in 34 patients and there was a hyperaemic trans-stent gradient across the edges of stent in five patients; after high-pressure balloon inflation, FFR increased to ≥0.96 in three patients and an FFR ≥0.96 was achieved in 35 patients (53%, group 1), but FFR remained <0.96 in 31 patients (47%, group 2). There was no correlation between FFR and minimum lumen diameter in group 1 or group 2 (r=0.03; p=0.72 and r=0.02; p=0.22, respectively). The 24-month event-free survival estimate defined as freedom from death, MI, and target vessel revascularisation (PCI or CABG) was significantly greater in group 1 than in group 2 (94% versus 72%, respectively; p=0.02).
Conclusions: After transradial stenting with predominately DES, an FFR ≥0.96 was achieved in only 53% of patients and event rates among patients with a post-stent FFR ≥0.96 were significantly lower than those with an FFR<0.96.
Introduction
It is well documented that intravascular ultrasound (IVUS) facilitates the assessment of stent expansion or adjacent plaque dislocation more accurately than angiography1. A number of series2-4 have shown that angiography alone is not a precise technique to detect local areas of incomplete stent expansion; in fact, 40 to 70% of deployed stents, which appear well expanded by angiography, but are not optimally expanded by IVUS3. Likewise, dislocation of plaque at the entry or the exit sites of stents is often not recognised by angiography4.
A number of series have shown that the measurement of fractional flow reserve (FFR) after stenting predicts adverse cardiac events at follow-up5,6. In this respect, it has been demonstrated5,6 that patients with a post-stent FFR >0.95 had low event rates, whereas those with an FFR <0.95 had increased event rates during follow-up.
It has also been shown that an FFR ≥0.96, after high-dose intracoronary adenosine, correlates well with an optimal stent expansion determined by IVUS7, however, the clinical correlate of which after drug-eluting stents (DES) has not been studied. In this respect, we sought to investigate the proportion of patients in whom an FFR ≥0.96 can be achieved after transradial stenting predominantly with DES and to determine their clinical outcomes based on a post-stent FFR ≥0.96 vs. an FFR<0.96.
Methods
Patient population
After transradial coronary angiography, 92 patients with single-vessel disease underwent FFR measurements. Of these, FFR was <0.75 in 66 patients and underwent transradial stenting. A total of 26 patients who had an FFR >0.75 were excluded from the study. The exclusion criteria were as follows: (1) recent myocardial infarction (<6 weeks); (2) unstable angina or the presence of haemodynamic instability; (3) distal vessels that were totally occluded; (4) patients with renal failure (serum creatinine >1.5 mg/dL); (5) patients with severe LV dysfunction (LVEF ≤30%); (6) patients with bifurcation lesions; and (7) patients with inadequate collateral supply of hand (negative Allen’s test). The institutional review board approved the study and informed consents were obtained.
Experimental protocol
All patients were pre-treated with aspirin and clopidogrel. Heparin (100unit/kg) was administered after the insertion of arterial sheath. Glycoprotein IIb/IIIa receptor inhibitors were not used in any of the patients. After engaging with a 6Fr guiding catheter without side holes, 100 mg intracoronary nitroglycerine was administered. A0.014-inch pressure guidewire (Wave Wire; Volcano, Rancho Cordova, CA, USA, or Pressure Wire; St. Jude Medical, Minneapolis, MN, USA) was used and FFR was calculated during maximal hyperaemia induced by intracoronary administration of adenosine (42µg to 54µg), as described previously8,9. After stenting, in patients in whom FFR was <0.96, the pressure transducer was positioned just to the distal and the proximal edges of the stents and FFR was measured at both locations. In patients in whom there was a hyperaemic trans-stent gradient (HTSG) across the edges of stents, further balloon inflation using a non-compliant balloon with an inflated diameter >0.5 mm than stent diameter was attempted.
Following transradial stenting, the arterial access sheath was removed immediately, and haemostasis was obtained by compression with a tourniquet. According to protocol, after stenting, patients in whom FFR was ≥0.96 were discharged on the same day after four hours of observation (group 1), whereas those with an FFR<0.96 (group 2) were admitted to hospital and discharged on the next day. A representative example of a patient from the group1 and the group 2 is shown in Figure1 and Figure2, respectively.
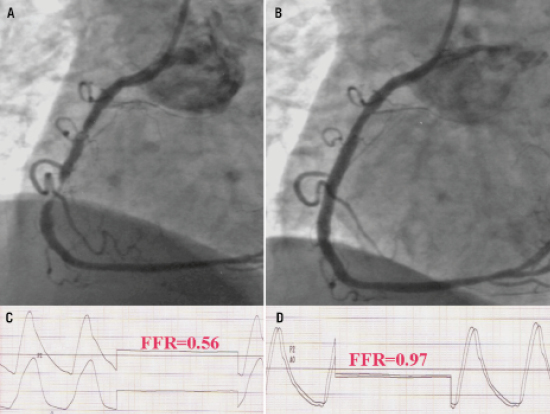
Figure 1. A representative example of a patient who underwent transradial stenting and FFR after stenting was 0.97. Panel A, coronary angiogram demonstrated a critical stenosis of the mid RCA, the baseline FFR was 0.56 (panel C); Panel B, after predilation with a 2.5 mm balloon, stenting of the mid RCA was performed with a 3.5·28 mm drug-eluting stent at 17 atm; final FFR was 0.97 (Panel D), the patient was discharged home on the same day and no event was noted during follow-up.
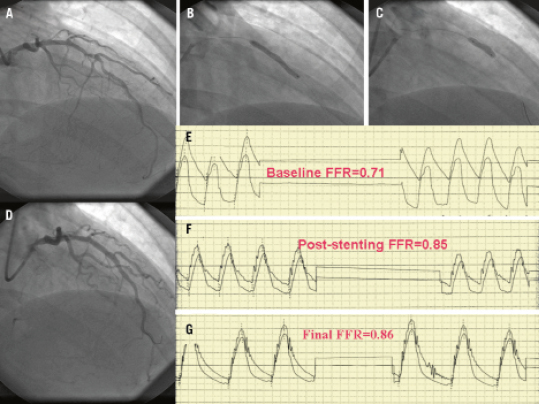
Figure 2. A representative example of patient who underwent transradial stenting; FFR after stenting was 0.86. Panel A, coronary angiogram demonstrated a stenosis of the mid LAD; the baseline FFR was 0.71 (panel E); panel B, direct stenting of mid LAD with a 3.0·28 mm drug-eluting stent was performed; panel F, post-stent FFR was 0.85; panel C, post-stent high pressure balloon inflation with a 3.5·15 mm non-compliant balloon was primarily performed in the mid and proximal parts of the stent; panel G, final FFR was 0.86. The patient was admitted to the hospital and discharged on the next-day. The patient was re- admitted seven months later with chest pain and repeat coronary angiogram revealed a significant in-stent restenosis located in the distal part of the stent. IVUS of the LAD demonstrated that the distal segment of the stent was under-expanded with a minimal lumen area of 3.4 mm2, which was treated a 3.5·16 mm drug-eluting stent.
Quantitative coronary angiography
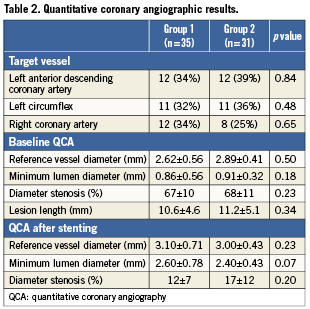
Quantitative coronary angiography (QCA) was performed by the use of automated edge-detection software (QCA-CMS 5.2 system; CMS-MEDIS, Nuenen, The Netherlands). The angiographic projection, demonstrating the most severe stenosis without foreshortening, was chosen to calculate standard angiographic parameters such as minimum lumen diameter (MLD), reference vessel diameter (RD), and percent diameter stenosis (%DS)8,9.
Follow-up and clinical events
Complete clinical follow-up was obtained from all the patients via personal contact during office visit and the review of medical records. Major adverse cardiac events (MACE) were defined as cardiac death, myocardial infarction (MI), or target lesion revascularisation (repeat PCI or CABG).
Statistical analysis
We estimated that the incidence of MACE in patients with a post-stent FFR ≥0.96, which correlated well with an optimal stent expansion, would be 5%, whereas the MACE rate in those with an FFR<0.96 with an incomplete stent expansion would be 30% (an 83% relative risk reduction); These estimates were based on the results of two registries that investigated the impact of post-stent FFR on MACE5,6. Using a two-sided alpha level of 5% and a power of 80%, we estimated at least 66 patients were required. Continuous variables between the two groups were analysed by the unpaired Student’s t test and categorical variables were analysed by the chi-square test. To identify correlations between FFR and minimum lumen diameter, logistic regression analysis was performed. Observed MACE rates were estimated using the Kaplan-Meier method. The event-free survival estimate of the patients with an FFR ≥0.96 vs. FFR<0.96 was compared using log-rank test. Apvalue <0.05 was considered significant.
Results
Clinical characteristics
FFR measurements and stenting was successful in all 66 patients. Based on post-stent FFR value, patients were divided into two groups: group 1, FFR ≥0.96 (35 patients); and group 2, FFR <0.96 (31 patients). The clinical features of the two groups are outlined in Table1. There were no significant differences between the two groups with respect to age, gender, or modifiable cardiac risk factors.
Procedural characteristics
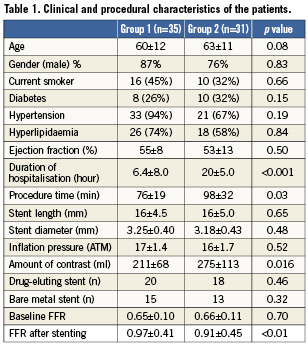
Direct stenting was successfully performed in 70% of patients. In the rest of patients, stenting was performed after predilation. FFR was significantly greater in group 1 than in group 2, p<0.01 (Table 1). In addition, after stenting, FFR was ≥0.96 in 32 patients (48%). Of the 34 patients in whom FFR was <0.96, there was a hyperaemic trans-stent gradient across the edges of stents in five patients; following high-pressure balloon inflation using a non-compliant balloon with an inflated diameter >0.5 mm than stent diameter, final FFR increased to ≥0.96 in three patients, and FFR remained <0.96 in others. Consequently, an FFR ≥0.96 was achieved in 35 patients (53%). These patients had no complications including no-reflow phenomenon, chest pain, or ECG changes, and were discharged on the same day of stenting (group 1). In contrast, 31 patients (47%), whose FFR remained <0.96, were admitted to hospital and discharged on the next day (group 2). The distribution of individual and average FFR values before and after stenting is depicted in Figure3. After stenting, there was no correlation between FFR and MLD as measured by QCA in group 1 or group 2 (r=0.03; p=0.72 and r=0.02; p=0.22, respectively).
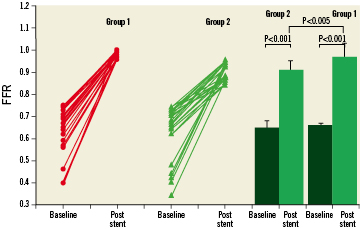
Figure 3. Individual (left panel) and average (right panel) values of FFR at baseline and post-stent deployment in the group 1 compared with the group 2 patients.
There were no differences between the groups with respect to inflation pressure, stent diameter, and stent length (Table1). The procedure time was significantly shorter in group 1 than in group 2. Similarly, the amount of contrast media used was significantly lower in group 1 than in group 2 (Table1). There were no differences between the groups with respect to MLD, RD, %DS, and lesion length at baseline and after stenting (Table2).
Clinical outcomes
Stenting was successfully performed in all patients; no patient died nor required emergency coronary artery bypass grafting.
The average follow-up was 24 months (range: 7-30 months). Irrespective of post-stent FFR value, no patient developed acute or subacute stent thrombosis. The follow-up and clinical outcome is summarised in Table3. In group 1, during follow-up period, one patient died because of throat cancer, and four patients were admitted with chest pain and underwent cardiac catheterisation. In two patients, because of in-stent restenosis in the BMS, DES were deployed.
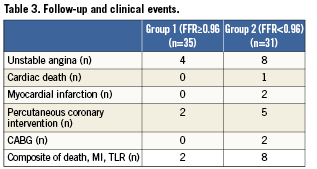
In group 2, during the follow-up period, two patients died because of cancer. In addition, one patient died suddenly 12 months after DES deployment; his post-stent FFR was 0.79. Furthermore, eight patients were admitted to hospital because of chest pain and underwent cardiac catheterisation. Two patients developed non-Q-wave MI after DES deployment; because of in-stent restenosis and progression of disease to the left main coronary artery, coronary bypass surgery was performed. In five patients, because of in-stent restenosis (three DES and two BMS) DES were deployed. The Kaplan-Meier event-free survival estimate at 24-month of follow-up was 94% in group 1 versus 72% in group 2, p=0.02 (Figure4).
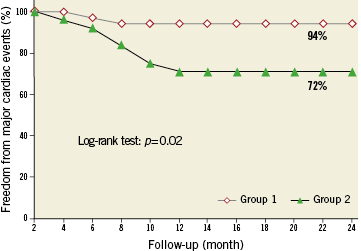
Figure 4. 24-month Kaplan-Meier freedom from major cardiac events in the group 1 compared with the group 2 patients.
Discussion
In the present study, we demonstrated that an optimal post-stent FFR, an FFR ≥0.96, was achieved in only 53% of patients and event rates among patients with a post-stent FFR ≥0.96 were significantly lower than those with an FFR<0.96. In addition, we showed that in patients with an FFR<0.96, there was a hyperaemic trans-stent gradient in five patients, but after further high-pressure balloon inflation, FFR increased to ≥0.96 in only three patients. Furthermore, we noted no incidence of acute or subacute stent thrombosis in patients with an FFR <0.96.
The present study extends the observation of Fearon et al study7 in which they demonstrated that a post-stent FFR ≥0.96 after intracoronary adenosine (30µg to 40µg) correlated well with an optimal stent expansion documented by IVUS. In the present study, such an improvement in clinical outcomes, among patients with a post-stent FFR ≥0.96, is likely linked to an optimal stent expansion. In addition, the present study corroborated the results of two registries in which the impact of post-stent FFR value on clinical outcomes was investigated. In this respect, Pijls et al5 demonstrated that patients with a post-stent FFR >0.95 had a low event rate of 4.9%, whereas those with a post-stent FFR ≤0.80 had an event rate of 29.5%. Likewise, Klauss et al6 reported that patients with a post-stent FFR <0.95 had event rates, which were about six fold greater than those with an FFR ≥0.95. However, the present study differs in some aspects from the above registries5,6. First, in the above registries, the final FFR results were correlated with clinical outcomes at follow-up, however, in the present study, the event rate was predicated on FFR cut-point of 0.96. Second, the above registries5,6 included all patients who underwent FFR measurements after stenting, whereas in the present study, patients with severe LV dysfunction (an EF ≤30%) and bifurcation lesions were excluded to offset the adverse effect of LV dysfunction and bifurcation lesions on clinical outcomes. Third, in contrast to the present study, in the above registries, a hyperaemic trans-stent gradient was not performed to elucidate the presence of hyperaemic trans-stent gradient. Furthermore, all patients in the present study underwent transradial stenting to eliminate bleeding complications as a confounding factor on clinical outcomes10-12.
In the present study, we found a hyperaemic trans-stent gradient in five patients with an FFR< 0.96 (16%) and that improved in three patients using a larger non-compliant balloon and high-pressure inflation. Likewise, others13-15 performed a slow pullback of the pressure guidewire during hyperaemia and demonstrated a small hyperaemic trans-stent gradient, which correlated with incomplete stent apposition by IVUS in one study13. In this respect, a slow pullback of the pressure guidewire during hyperaemia will not only determine an abnormal conductance within the stented segment but also elucidate increased plaque accumulation in the remaining part of the coronary artery, which can account for a reduction in FFR value14,16. It is worth nothing that in the present study, an FFR<0.96 was linked to trans-stent gradient in five patients; however, in others, the underlying mechanism for suboptimal FFR result is likely related to the presence of diffuse disease in the segments proximal or distal to the stent.
In the present study, the decision, as to whether to discharge those patients with an FFR ≥0.96 on the same day, was based on the Fearon et al7 study that showed patients with an FFR ≥0.96 had optimal stent expansion. In this respect, we determined that such patients can be safely discharged on the same-day. In contrast, Klauss et al6 reported that one patient in their series with a post-stent FFR of 0.79 developed subacute stent thrombosis. Based on such an adverse event, we admitted those patients with a suboptimal FFR result for overnight observation. However, we noted no incidence of acute or subacute stent thrombosis. Since the incidence of acute stent thrombosis in the current era of stenting is rare, a large study may determine the safety of same day discharge based on post-stent FFR ≥0.96 vs. an FFR<0.96. On the other hand, it has been reported17-20 that patients, who had favourable clinical and angiographic criteria after transradial stenting and discharged after an observation period of four to six hours, had no incidence of acute stent thrombosis17-20. Taken together, it seems that a favourable clinical and angiographic result after stenting is a reasonable strategy for decision-making on the same day discharge.
Limitations of the study
This was an exploratory trial that was designed to provide preliminary data and to generate hypothesis for future studies. In the present study, a post-stent FFR was <0.96 in 47% of patients. We measured FFR at the distal and proximal edges of stents after intracoronary adenosine administration and found the presence of hyperaemic trans-stent gradient in five patients, which resolved after high-pressure balloon inflation in three patients with a parallel increase in FFR to ≥0.96. Since we used intracoronary adenosine instead of intravenous adenosine, we could not definitely determine the underlying mechanism of suboptimal FFR in our patients. In this respect, in patients with an FFR <0.96, the use of a pressure pull-back curve induced by intravenous adenosine would have distinguished underlying mechanisms responsible for suboptimal FFR results such as abnormalities within the adjacent segments to the stent, or diffuse disease more proximal or distal to the treated segments. The use of IVUS, in particular, in patients with a persistent trans-stent gradient, could have further delineated the underlying cause of suboptimal FFR results such as stent underexpansion, plaque protrusion, or edge dissection. In addition, it was expected that patients with an FFR<0.96 would have more diffuse disease. However, using interpolated QCA analysis, we analysed only the tightest segment of artery and lesions located more proximal or distal to the artery were not analysed because it would have compromised the automatic measurements of the stenosis severity by QCA analysis. Another limitation of the study is that we did not use DES in all patients to eliminate the confounding effect of restenosis associated with BMS.
Conclusions
We demonstrated that a post-stent FFR ≥0.96 was achieved in 53% of patients and events rates during follow-up were significantly lower among patients who had an FFR ≥0.96 compared with those with an FFR<0.96. Given the prognostic significance of a post-stent FFR ≥0.96, it is unknown what proportion of suboptimal post-stent FFR results would improve after further intervention guided by pressure pullback curves during hyperaemia or intravascular ultrasound.
Conflict of interest
All authors have no conflicts of interest to declare.

