Abstract
Percutaneous coronary intervention (PCI) of coronary artery bifurcation lesions entails technical challenges and carries a higher risk of adverse events on follow-up, driven by repeat revascularisation and stent thrombosis. While most bifurcations can be tackled with a provisional (single-stent) approach, more complex lesions involving both branches (true bifurcation lesions) require a two-stent approach. In the latter context, several techniques have been proposed. Among them, the crush technique has dramatically evolved in recent years, and its more recent iterations have been shown to provide excellent and durable results, both for left main and non-left main bifurcations. The aim of the present work is to discuss the technical aspects and outcomes of the variants of the crush technique from the first description in the early 2000s to the present day.
Introduction
Significant atherosclerosis of coronary artery bifurcations is a common finding among patients undergoing coronary angiography12. True bifurcation lesions, i.e., those with significant stenosis in both the main branch (MB) and side branch (SB)3, account for 2-16% of lesions considered for intervention12. Approximately 60-70% of lesions affecting the left main (LM) involve the distal bifurcation4. Percutaneous coronary intervention (PCI) of bifurcations is challenging, and has been associated with higher rates of complications on short- and long-term follow-up5, mainly driven by target lesion revascularisation6. Bifurcation treatment also has the potential of SB occlusion, which has been associated with periprocedural myocardial infarction7. Several techniques have been proposed for bifurcation PCI5. The single-stent technique, also called the “provisional strategy”, is recognised as the standard approach for the majority of bifurcations, particularly when only one of the branches is stenotic or when the risk of SB occlusion or difficulty in SB bail-out management is considered low8. Two-stent techniques, which involve stent deployment in both the MB and the SB, allow bail-out treatment of suboptimal results after a single-stent approach. Furthermore, an upfront two-stent strategy in high-risk bifurcations, termed "complex bifurcations", provides a significant advantage in terms of major adverse cardiac events (MACE) when compared to a single-stent strategy910. The most commonly adopted criteria to define a bifurcation complex are derived from the Definitions and impact of complEx biFurcation lesIons on clinical outcomes after percutaNeous coronary IntervenTIOn using drug-eluting steNts (DEFINITION) registry, and include LM bifurcation with SB stenosis ≥70%, non-LM bifurcation and SB stenosis ≥90%, presence of significant calcification, multiple lesions, narrow bifurcation angle, long atherosclerotic lesions, and presence of thrombus10. In light of these considerations, knowledge and optimal implementation of two-stent techniques is critical when approaching bifurcations. Several different techniques are currently available. The ideal two-stent approach should allow perfect coverage of the bifurcation including the SB ostium without carina shift, avoid floating stent struts in the vessel lumen, be easy to perform by most operators, offer reproducible results across diverse anatomies, and be compatible with 6 French (Fr) guide catheters. The perfect technique does not, in fact, exist, and each technique has its shortcomings. Simultaneous kissing stenting and V-stenting are generally associated with the formation of a long metallic neocarina (with the former) or a high risk of missing the SB ostium (with the latter). Y-stenting requires at least three stents for adequate bifurcation coverage, and an 8 Fr guide8. The T and small protrusion (TAP) technique requires a bifurcation angle ~90 degrees, and carries the risk of either incomplete stent coverage of the SB ostium or excessive protrusion into the proximal MB, leading to the formation of a relatively long metallic neocarina, which can hamper further access with balloons and stents through the MB11. Culotte techniques are limited to bifurcations with similar diameter of the MB and SB and imply a double layer of metal throughout the MB circumference for a significant length8. On the other hand, crush techniques, which involve upfront stenting of the SB with minimised protrusion into the proximal MB, consistently allow full coverage of the SB ostium across a wide spectrum of take-off angles, and can ensure upfront protection of the SB when the latter is at risk of compromise if a provisional technique were to be utilised. The aim of the present review is to provide a critical appraisal of the evolution of the crush techniques, their strengths, and data regarding their implementation in clinical practice. Table 1 highlights the main differences between the different iterations of the technique, while the Central illustration reports the historical evolution of and provides a step-by-step guide to the crush techniques.
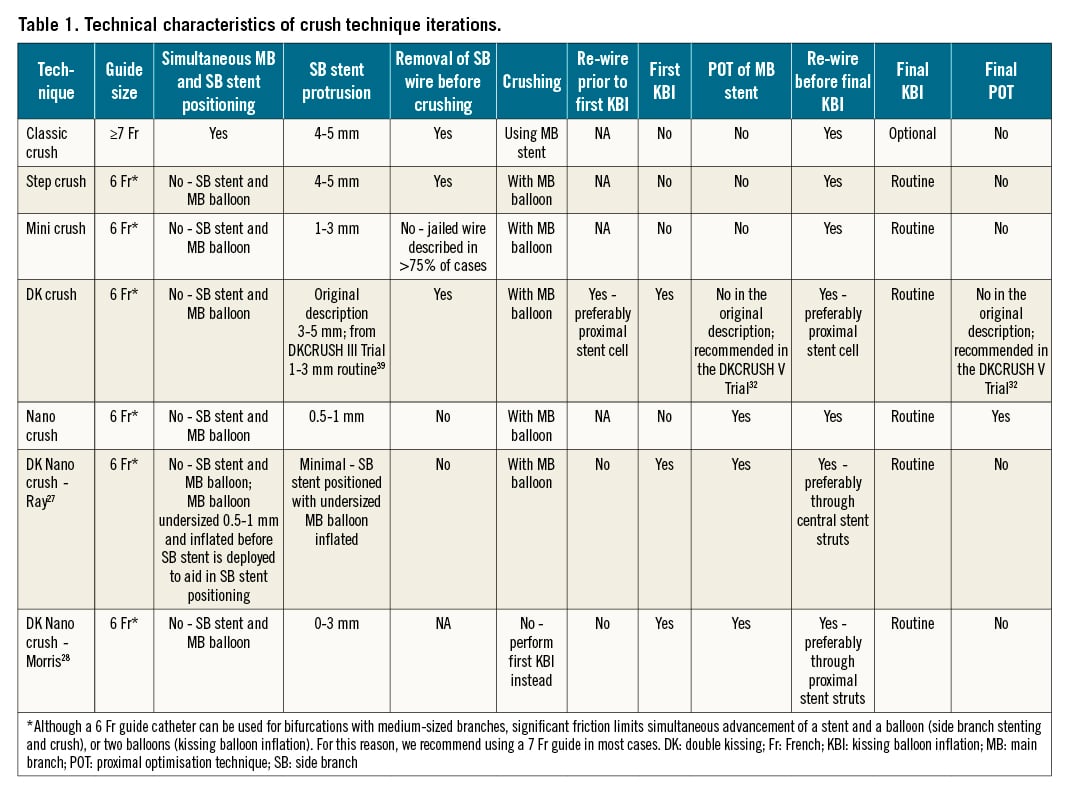
Central illustration. Schematic of different crush techniques. The top panel presents the timeline of publication of each technique. A) Classic crush. B) Step crush (red arrows) and double kissing (DK) crush (blue arrows). C) Contemporary versions of the classic crush (black arrows), mini crush (red arrows), and DK crush (blue arrows). Note: the proximal optimisation technique (POT) is advocated in the latest iterations of the DK crush technique. D) Nano crush (red arrows) and DK nano crush (blue arrows). Note: POT is performed in both iterations. KBI: kissing balloon inflation; SB: side branch
CLASSIC CRUSH
Colombo and colleagues first reported the crush technique, referred to here as the “classic crush”, in a single-centre case series of 20 subjects in 200312. Evidence leading up to that time showed that, even with the advent of drug-eluting stents (DES), in-stent restenosis (ISR) of bifurcation lesions was still observed in one fourth of patients13. Importantly, most of the ISR cases (79%) with two-stent approaches using DES were localised in the SB ostium, which was thought to be related to incomplete stent coverage13. The crush technique was thus developed to minimise the chance of incomplete coverage of the SB ostium. In its original description, both the MB and the SB are wired and predilated sequentially. A first stent is advanced into the SB but not expanded, and a second stent is subsequently advanced into the MB. The SB stent is subsequently retracted so that the proximal marker is located in the proximal MB, approximately 4-5 mm from the carina, in order to ensure complete coverage of the SB ostium. The MB stent is positioned across the SB ostium and the entire protruded segment of the SB stent. The SB stent is then deployed, and the stent balloon removed. After contrast injection to evaluate the absence of SB stent distal edge dissection, the wire is also removed. The MB stent is deployed, crushing the protruding struts of SB stent against the MB wall. This way, there are three layers of stent struts in the proximal margin of the bifurcation and SB ostium, but no floating struts in the vessel. SB re-wiring and final kissing balloon inflation (KBI) are not advocated unless the final angiographic result is unsatisfactory. The classic crush aimed to represent a simplified approach to address the issue of missed/incomplete stent coverage of the SB ostium, responsible for the high rates of ISR localised to the SB ostium observed in T-stenting techniques. It also avoids difficult SB stent delivery through the MB stent. However, the rate of SB ISR did not improve with classic crush, because systematic final KBI was not used. A larger study of the classic crush technique with nine-month follow-up showed that the rate of SB ISR (75% localised to the SB ostium) was 37.9% vs 11.1% without and with KBI, respectively14. Bench testing showed that SB stent struts are actually malapposed (particularly in larger-angle bifurcations, e.g., >45°) at the distal margin of the SB ostium by the crush process, causing incomplete coverage of the SB ostium. This can be corrected by full expansion of the SB ostium with KBI15. Major disadvantages of this technique are that: (1) re-crossing into the SB for final KBI is generally more difficult after deployment of the MB stent compared to other techniques, due to there being two layers of stent struts at the SB take-off (this step was originally deemed optional, which made the classic crush technique look easier to perform than other two-stent techniques); and (2) it requires large guide catheters (≥7 Fr) to accommodate two stents simultaneously (at the time of the original description, 8 Fr guides were required), making a transradial approach challenging in patients with small radial arteries. Figure 1 shows a case of a left anterior descending artery – first diagonal branch bifurcation treated with a contemporary classic crush approach.
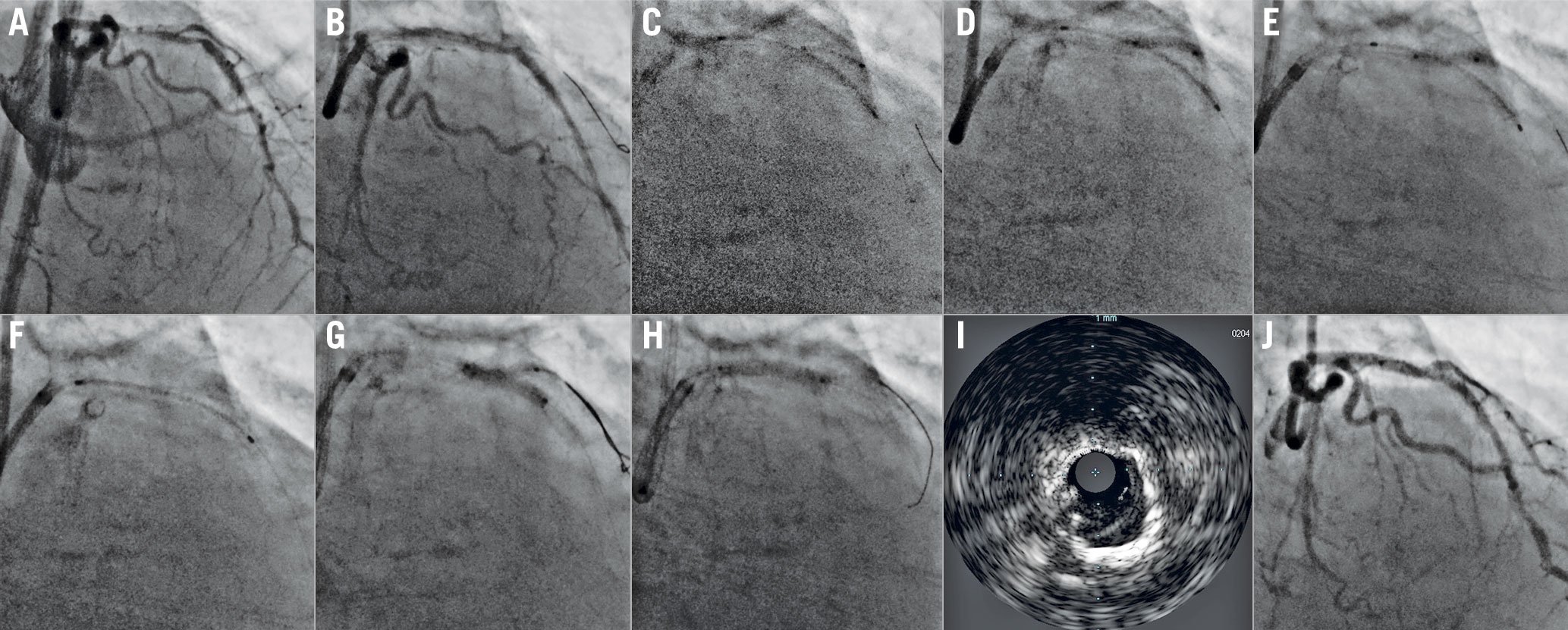
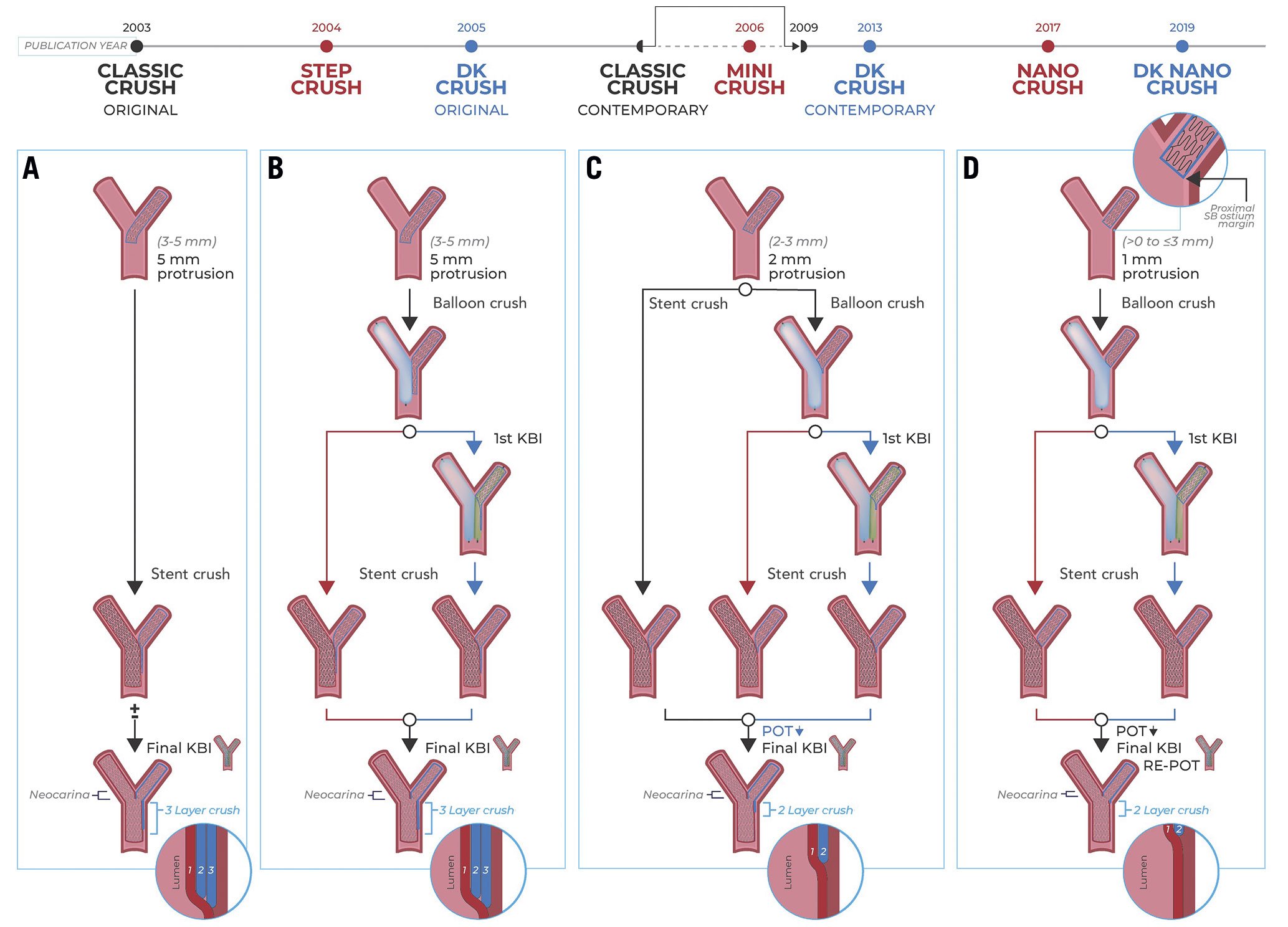
Figure 1. Contemporary classic crush treatment of a left anterior descending – first diagonal bifurcation (LAD-D1). A) LAD-D1 complex bifurcation (Medina 1,1,1, severely calcified LAD). B) After rotational atherectomy of the LAD, D1 suffers acute vessel closure. Both branches are wired, and vessels are predilated, thus restoring flow in D1. C) The D1 stent and LAD stent are inserted via the 7 Fr guide, with the D1 stent protruding approximately 2 mm into the LAD, and the LAD stent positioned across the bifurcation. D) The D1 stent is deployed, and E) its balloon is pulled back partially into the LAD and inflated at rated burst pressure to optimise stent apposition/expansion at the D1 ostium. F) D1 stent balloon and wire are removed, and the LAD stent is deployed. G) D1 is rewired, and kissing balloon inflation is performed with two non-compliant balloons. H) Finally, the proximal optimisation technique (POT) is performed in the proximal LAD stent. I) Intravascular ultrasound, and (J) angiography show an excellent final result.
STEP CRUSH
In 2004, Lim and Dzavík described a modification of the classic crush technique allowing, with the use of smaller guide catheters (6 Fr), for a transradial approach, in a single-centre case series of seven subjects16. In this modified technique, called "step crush" (original term: balloon crush), only “one stent plus one balloon” (SB stent and MB balloon) instead of two stents (SB stent and MB stent) need to be delivered via the guide catheter at the same time16. In the step crush technique, after wiring the two branches of the bifurcation, a stent is advanced into the SB, while a balloon is positioned in the MB across the SB ostium. The stent is subsequently positioned to extend into the MB for approximately 4-5 mm, as in the classic crush technique. After SB stent deployment, the SB stent balloon and wire are removed, and the MB balloon is inflated to crush the protruded struts of the SB stent. Subsequently, a stent is positioned in the MB and deployed. Finally, the SB is rewired and final KBI is performed16. Compared to the classic crush technique, the step crush has the advantage of being 6 Fr guide catheter-compatible (although a 7 Fr guide is recommended for bifurcations of large vessels), thus allowing the transradial approach in the vast majority of patients. However, both techniques share the same disadvantage of potential difficulties with SB re-access for final KBI. Nevertheless, this can be mitigated as it provides the opportunities: (1) to open the SB stent struts at the ostium (and improve stent expansion/reapposition at the SB ostium) prior to MB stenting, thus increasing the chance of successful SB re-access post-MB stenting for KBI; and (2) of a more precise positioning of the MB stent prior to its deployment17. Figure 2 shows an example of the step crush technique.
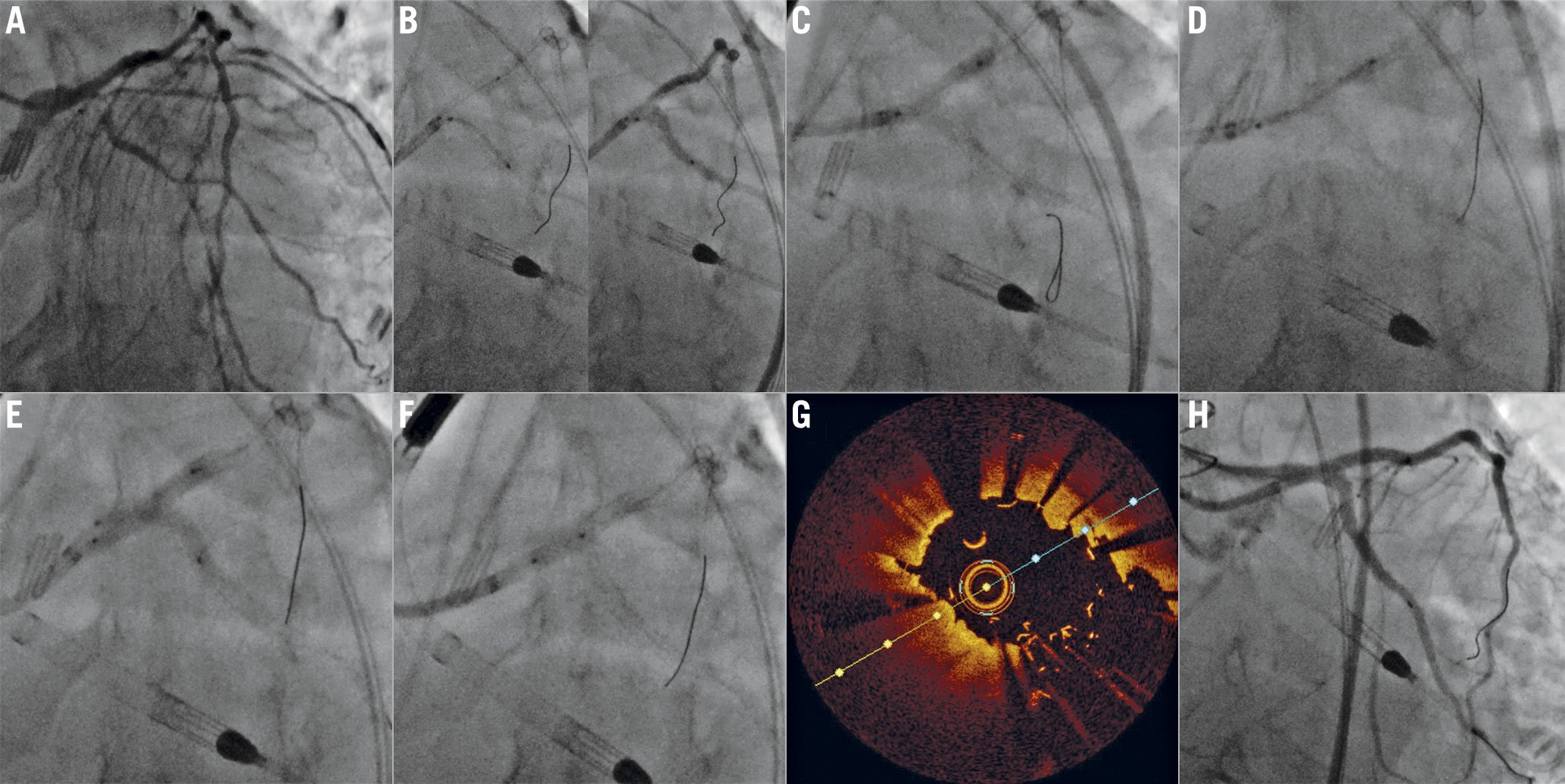
Figure 2. Step crush treatment of the left main (LM) bifurcation. A) Complex bifurcation lesion of the LM (Medina 1,1,1, severe calcification in both the left anterior descending [LAD] and left circumflex [LCx]). Both branches are wired and treated with rotational atherectomy, and then predilated. B) A stent is positioned in the LCx, protruding ~1-2 mm into the LM, while a balloon is positioned in the LAD across the bifurcation. The LCx stent is deployed and, after removing its balloon, C) it is subsequently crushed using the LM-LAD balloon. D) The LCx wire is removed, and the LM-LAD stent is deployed. E) The LCx is rewired, and kissing balloon inflation is performed, F) followed by the proximal optimisation technique. G) Final optical coherence tomography shows excellent apposition and expansion of the LM-LAD stent at the LCx ostium, with a short and symmetric neocarina. H) Final angiogram also shows an excellent result.
DOUBLE KISSING CRUSH
In the double kissing (DK) crush technique, final KBI is a key step that corrects for stent distortion, namely: (1) malapposition of the crushed SB stent struts at the distal margin of the SB ostium15; (2) protrusion of the crushed SB stent struts into the MB lumen at the level of the SB ostium caused by SB balloon inflation15; and (3) suboptimal carina geometry from isolated balloon inflation of the distal MB or SB. The use of MB and SB balloons sized 1:1 for final KBI is required to correct for these stent distortions. However, SB re-access for final KBI after a crush is not straightforward. In the classic crush and step crush techniques described above, the wire needs to negotiate through two layers of stent struts: one of the MB stent and one of the crushed SB stent. Chen and co-workers18 and Jim and colleagues19 independently described a modification of the classic crush technique to increase the success of SB re-access for final KBI to correct the stent distortion at the bifurcation mentioned above. In this modified technique, known as the "DK crush technique", the step crush approach was modified by the addition of a KBI just before MB stenting (first KBI)1819. In the DK crush technique, both the MB and the SB are wired, a stent is positioned in the SB extending 3-5 mm into the MB and a balloon (sized 1:1) is positioned in the MB. After deployment of the SB stent, the SB stent balloon and wire are removed and the balloon in the MB is inflated, crushing the SB stent. At this point, the SB is rewired and a first KBI is performed. The SB balloon and wire are removed, and a stent is introduced and deployed in the MB across the bifurcation. The SB is subsequently rewired and final KBI is performed18. In later iterations of the technique, the use of the proximal optimisation technique (POT) was recommended to facilitate SB rewiring before the final KBI, or, if performed after the final KBI, to optimise proximal MB stent geometry (from oval to round)20. Chen et al compared 20 patients treated with DK crush with 20 patients treated with classic crush, and found that successful re-crossing of the SB and final KBI was 100% in the DK crush arm versus 80% in the classic crush arm18. Jim et al also reported a 100% success rate in SB re-access for final KBI in their 6-case series19. In successive iterations of the technique, protrusion of the SB stent into the MB was reduced from 3-5 mm19 to 1-3 mm21, which decreases the length of the triple stent layer in the MB at the proximal margin of the SB ostium. Moreover, if the protrusion length of the SB stent is less than the diameter of the SB ostium, the overlapping stent layer can be reduced from 3 to 2 layers. The first KBI allows full expansion of the SB ostium and reduces 2 layers of stent struts to 1 layer at the SB entrance after MB stenting across it. These make subsequent re-access of the SB easier, increasing the likelihood of success of the final KBI. Chen et al8 recommended re-wiring the SB through the proximal stent cell before the first KBI, to avoid wire passage under the stent struts of the malapposed/crushed SB stent at the distal margin of the SB ostium, because this would: (1) worsen stent malapposition (leaving an uncorrectable significant gap at the distal margin of the SB ostium) when performing subsequent SB ballooning/KBI, thus causing incomplete stent coverage of the SB ostium; and (2) make SB re-access with wires/balloons very difficult18. In the latest iteration of the technique, SB re-wiring through a “non-distal” stent cell is recommended. On the other hand, rewiring of the SB through a distal strut is advocated before the final KBI, in order to minimise neocarina length22. Adequate wiring is generally confirmed with two orthogonal angiographic projections, and ideally with intravascular imaging. Figure 3 shows an example of the DK crush technique. Due to reduced stent distortion with improved stent expansion/apposition and maintenance of carina geometry, final KBI markedly reduces the rate of ostial SB ISR (from 33.3% to 7.7-13.3%), stent thrombosis (from 5.1% to 1.3-1.7%), and MACE (from 35.9% to 11.4-19.7%)1423. This reduction was greater in DK crush compared with classic crush with final KBI, presumably due to an increased rate of effective correction of stent distortion at the bifurcation with the first KBI1423. A series of landmark trials has documented the efficacy of the DK crush technique, which reached a IIb recommendation for treatment of true bifurcations in the left main coronary artery in the 2018 European clinical guidelines for revascularisation24.
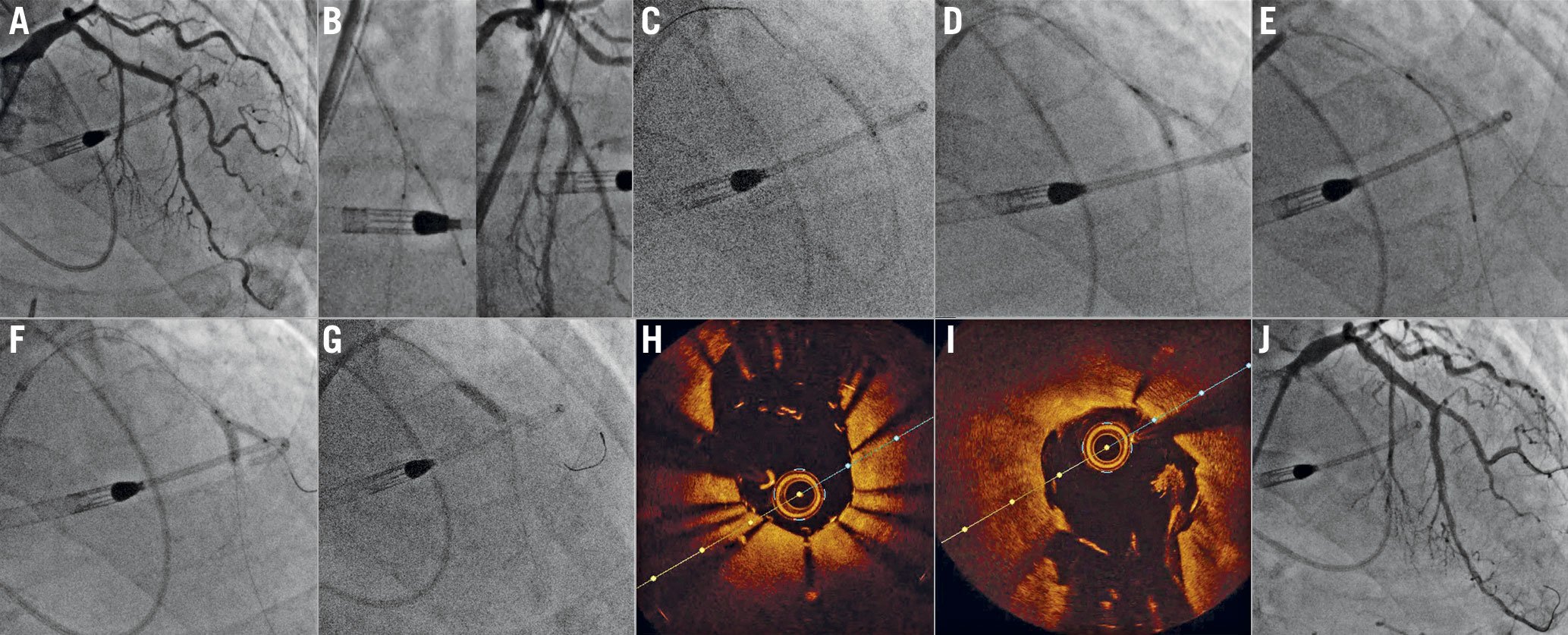
Figure 3. Double kissing (DK) crush of a left anterior descending – first diagonal (LAD-D1) bifurcation. A) Baseline angiogram shows a complex (Medina 1,1,1, severely calcified) LAD-D1 bifurcation. Both vessels are treated with aggressive rotational atherectomy and predilatation. B) A stent is positioned in D1, protruding approximately 1-2 mm into the LAD, while a balloon is positioned into the LAD. C) The D1 stent is deployed and crushed after removing its balloon and wire. D) After D1 rewiring, a first kissing balloon inflation is performed using non-compliant balloons. E) A stent is positioned in the LAD across the D1 ostium and deployed. F) D1 is re-wired, and a second kissing balloon inflation is performed (again with non-compliant balloons), G) followed by the proximal optimisation technique. Optical coherence tomography showed optimal stent expansion and short, symmetric neocarina in both H) D1 and I) LAD. Note a small thrombotic apposition at 3 o’clock on the LAD, I) with no clinical implications. J) Final angiogram showing an excellent final result.
MINI CRUSH
In 2006, Galassi and colleagues proposed a variation of the original crush, named the “mini crush” technique25, which is similar to the step crush technique except for the essential difference of reduced SB stent protrusion into the MB from 4-5 mm to 1-2 mm. An additional, but minor, difference is that the SB wire is not routinely removed prior to MB stent deployment (jailed wire technique used in 75% cases) with the mini crush technique25. In the mini crush technique, the stent in the SB is positioned extending just 1-2 mm into the MB, and is subsequently crushed with a balloon positioned in the MB (prior to SB stent deployment). The MB stent is then delivered and deployed, jailing the SB wire. The SB is re-wired (the jailed SB wire, where present, is then removed), and final KBI is performed. In the single-centre study (45 patients, 52 lesions) reported by Galassi and colleagues, final KBI was performed in 94.2%. The restenosis rate was only 2% in the SB, 12.2% in the MB and the rate of MACE 15.5% at 7.5±1.3 months25. It is likely that minimising the extent of protrusion of the SB stent reduces stent distortion and the number of layers of stent struts at the SB ostium, and allows easier SB re-access for final KBI. Interestingly, such an approach has been adopted in later iterations of the DK crush technique. It remains to be determined whether the first KBI in DK crush is still necessary when the SB stent protrusion into the MB is minimised to 1-2 mm. No clinical studies comparing the mini crush against DK crush techniques have been reported to date.
NANO CRUSH
In 2017, Rigatelli and co-workers reported the "nano crush" technique. This is essentially a modification of the mini crush technique, whereby the objective is to reduce the SB stent protrusion into the MB further, while preserving coverage of the SB ostium. This further minimises the amount of crushed metal mass around the margins of the SB ostium, and the length of double-layer stent struts in the proximal margin of the SB ostium26. In the nano crush technique, the SB stent is deployed extending in the MB by approximately 0.5-1 mm, while a deflated balloon sized 1:1 with the main branch is positioned across the distal MB. After removing the SB stent balloon, the SB stent is crushed by inflating the MB balloon. Then, the MB stent is deployed, and POT is subsequently performed. The side branch is then rewired, and KBI is performed. A final POT is performed to re-establish a round shape in the proximal MB stent26.
DOUBLE KISSING NANO CRUSH (DK NANO CRUSH)
The "DK nano crush" was recently developed to combine the perceived advantage of the DK crush in terms of SB ostial and carina geometry and the reduced number of overlaying struts at the level of the SB provided by the mini crush. Also, avoidance of a double layer of stent struts over the proximal SB ostium allows easy wire re-crossing after POT and a high success rate of KBI. Two variants of the technique have been reported2728. The first was described in 2019 by Ray and colleagues27. After adequate lesion preparation, a stent is advanced into the SB and a non-compliant balloon is advanced into the MB across the bifurcation. Care should be taken in selecting a balloon which is undersized with respect to the MB by ~0.5-1 mm. The balloon in the MB is inflated at nominal pressure. The SB stent is retracted towards the inflated balloon and deployed. Both balloons are then deflated, and the SB stent balloon is pulled back partially into the MB and inflated at high pressure (flared) to optimise expansion and apposition at the SB ostium. It is then deflated. The stent is subsequently crushed by high-pressure inflation of the MB balloon, which is then deflated. Subsequently, the first KBI is performed with the same balloons. The SB stent and wire are removed. The MB is stented, and POT is performed. The SB is then rewired through a central cell of the MB stent at the SB ostium, and the second KBI is performed (with non-compliant balloons)27. Figure 4 shows an example of the DK nano crush technique.
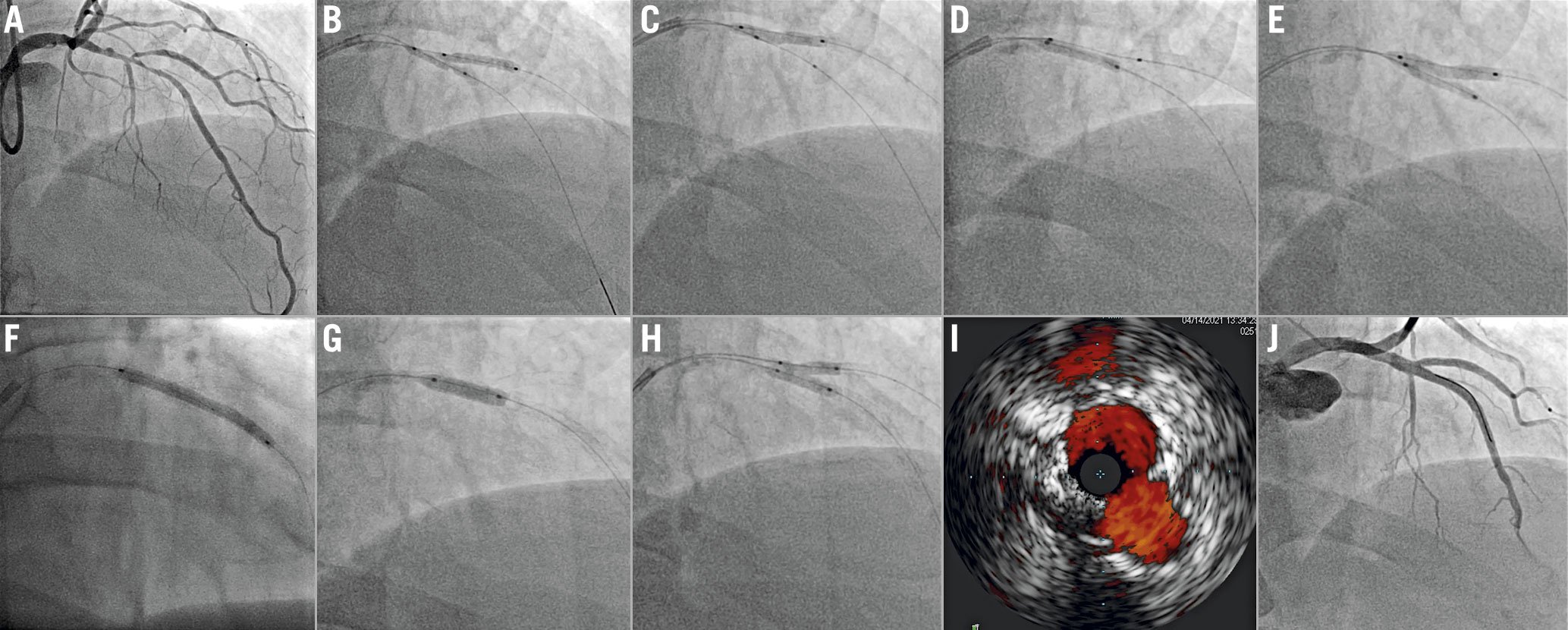
Figure 4. Double kissing (DK) nano crush of a left anterior descending – first diagonal (LAD-D1) bifurcation. A) Complex LAD-D1 bifurcation lesion (Medina 1,1,1). Both branches are wired and predilated. B) A stent is positioned into D1, while a non-compliant balloon sized approximately 1 mm less than the LAD diameter is advanced in the LAD and positioned across the bifurcation. This balloon is inflated. The D1 stent is pulled back towards the LAD to ensure minimal protrusion and is deployed. C) The D1 stent balloon is then pulled into the LAD and inflated at high pressure to ensure optimal stent expansion/apposition at the D1 ostium. D) The D1 stent is crushed with the LAD balloon, E) then the first kissing balloon inflation is performed with the D1 stent balloon and the LAD non-compliant balloon. F) D1 balloon and wire are removed, and the LAD is stented. G) After the proximal optimisation technique, H) the D1 is rewired and final kissing balloon is performed. I) Final intravascular ultrasound shows optimal stent expansion and a symmetric neocarina. J) Angiography shows an excellent final result.
The second variant was described in 2020 by Morris and colleagues28. In this case, the SB stent is deployed with minimal protrusion into the MB while the main branch balloon is positioned uninflated across the side branch ostium; the first KBI rather than the MB balloon is used to crush the protruded SB stent. The SB is rewired through a proximal cell of the MB stent at the SB ostium. The rest of the procedure is similar to the technique described by Ray et al27. Importantly, SB stent protrusion into the MB is necessarily lengthened as the bifurcation angle narrows, thus the minimum SB stent protrusion can be up to 3 mm for angles of less than 50° in practice (Figure 5)28.
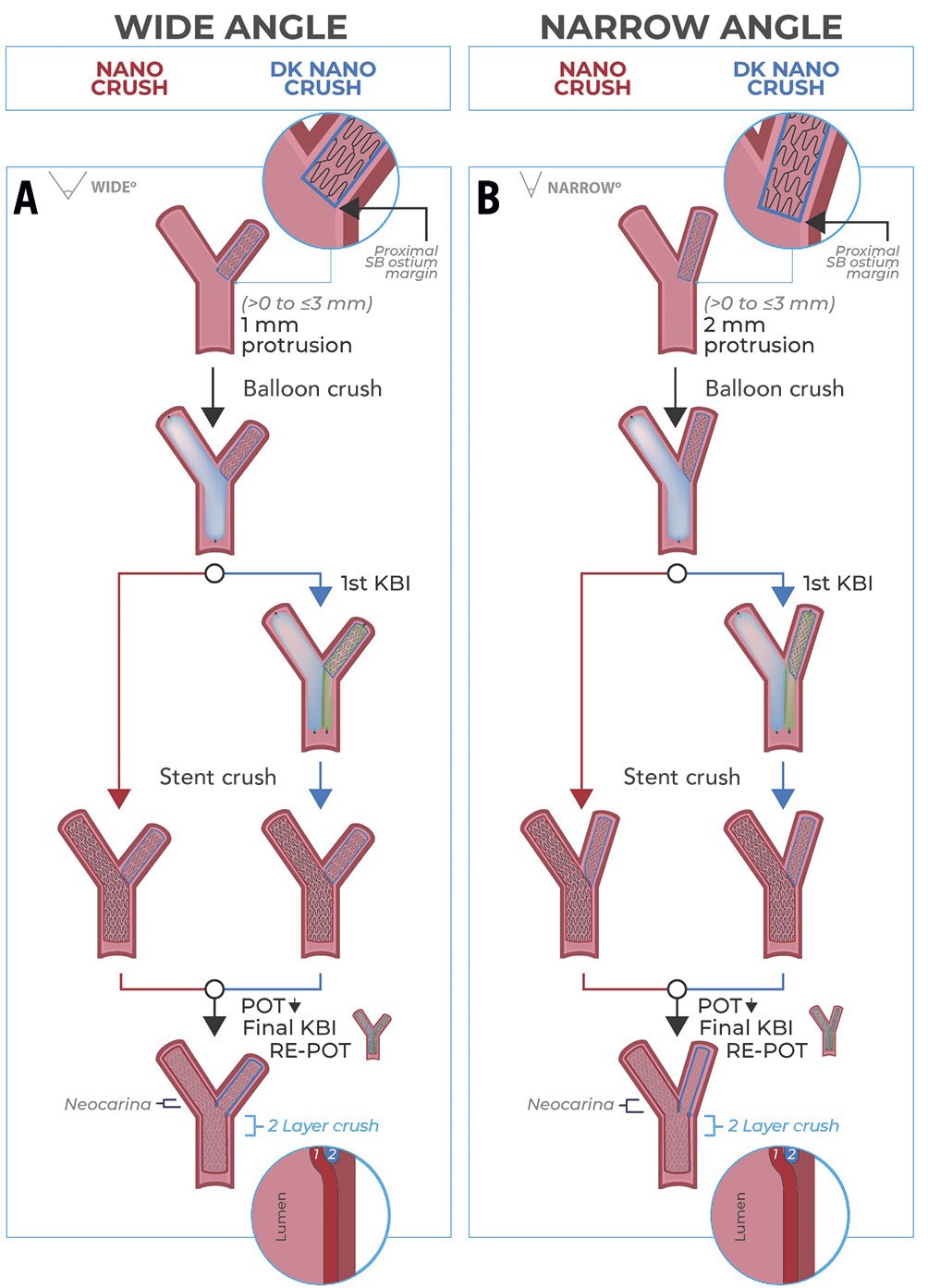
Figure 5. Impact of bifurcation angle on the length of protrusion of the SB struts into the MB. A) Bifurcation with a wide angle. The wider the angle, the smaller the protrusion into the MB. B) Bifurcation angle with a narrow angle, which invariably leads to increased protrusion into the proximal MB to ensure full SB ostium coverage. DK: double kissing; KBI: kissing balloon inflation; MB: main branch; POT: proximal optimisation technique; SB: side branch
A major disadvantage of all nano crush techniques is the possibility of incomplete stent coverage of the proximal margin of the SB ostium (and thus incomplete SB ostial coverage), with a minimal margin for error. To guide SB stent positioning it is of critical importance to optimally visualise the bifurcation while avoiding superimposition of other vessels. This should be achieved utilising multiple angiographic projections. While some projections are preferred for specific bifurcations (steep right anterior oblique [RAO] cranial for left anterior descending-diagonal, anterior-posterior [AP] caudal for circumflex-marginal, “spider” for the left main, left anterior oblique [LAO] cranial for the right coronary artery crux), a large variability is observed across different individuals29. If available, a pre-PCI computed tomography angiography can aid in the identification of the optimal projection to visualise the SB ostium during the preprocedural stage30. Where stent protrusion is more than intended, the procedure becomes de facto a contemporary DK crush.
TROUBLESHOOTING
As noted above, final KBI is a key step in all crush techniques. However, the presence of multiple layers of stent struts and the deformation of cells might represent a challenge for SB rewiring and the advancing of balloons. To wire the SB successfully, an algorithmic approach has been suggested. First, the wire tip may be reshaped to add or increase a secondary curve in order to facilitate engagement in the SB ostium. In rare cases of unfavourable SB take-off angles, the “hairpin” or reverse wiring technique might be required22. If wiring with a workhorse is unsuccessful, exchanging for polymer-jacketed guidewires or specialty wires with increased torqueability may be useful. The use of a microcatheter may increase wire support and allow easier reshaping and wire exchanges. For very angulated SB take-off angles, dual lumen, angulated or steerable microcatheters might be required. Finally, POT in the MB might induce modification of the geometry of the carina, which can facilitate rewiring. After rewiring, balloon crossing can also be challenging. If unable to cross with an appropriately sized balloon, we recommend using ultra-low-profile balloons (diameter ≤1.0 mm). Additional manoeuvres to be contemplated include: dilating the stent struts at the SB ostium with a microcatheter; coaxial catheter alignment, deep guide engagement, and guide catheter extensions to increase support; exchanging the SB wire for an extra-support guidewire using a microcatheter; making sure that the MB and SB wires are not intertwined (this requires pulling one wire out and rewiring); rewiring the SB through a different cell at the SB ostium level.
CLINICAL EXPERIENCE WITH THE CRUSH TECHNIQUES
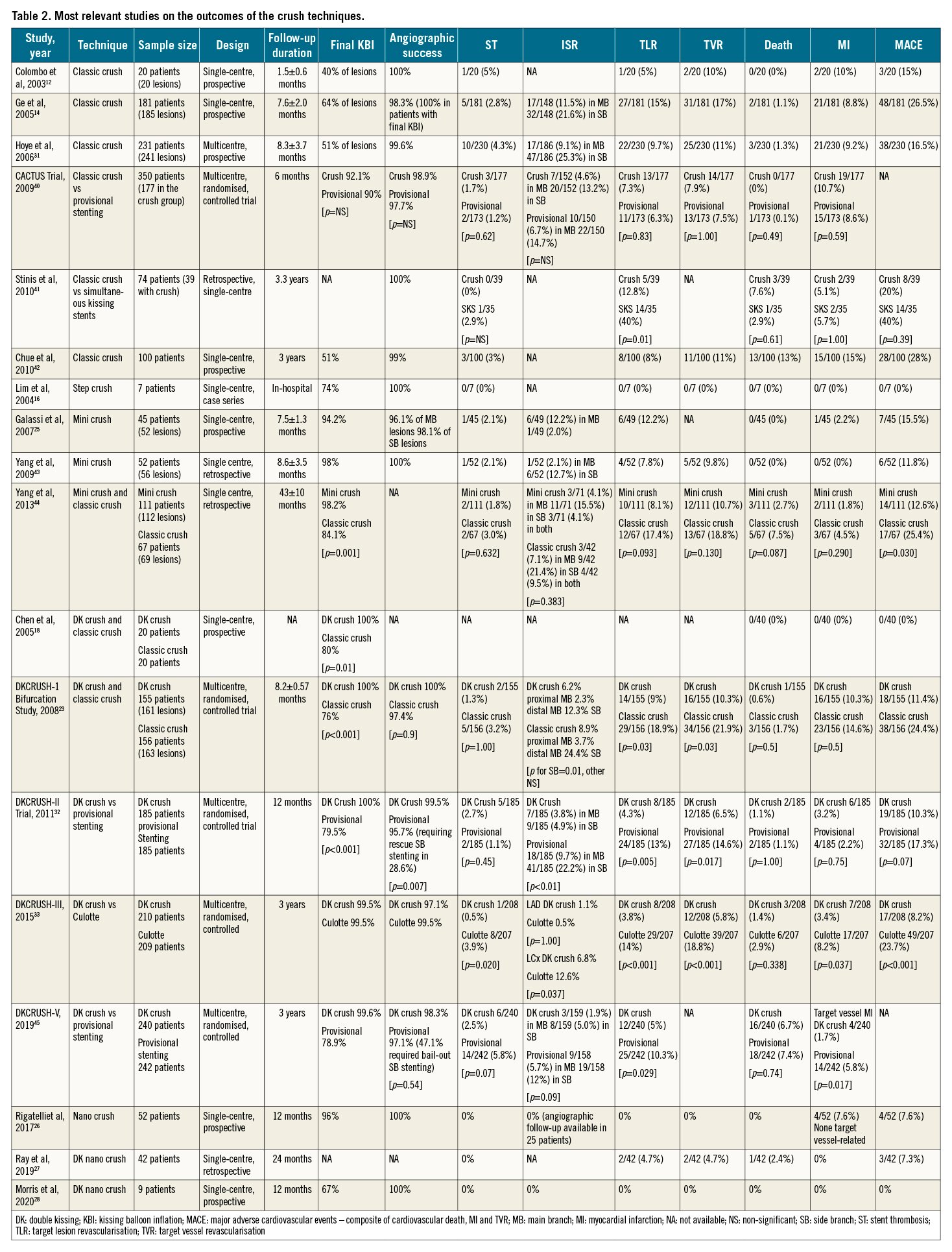
In the first report on the classic crush technique, Colombo et al reported angiographic success in all 20 patients treated12. However, a subsequent multicentre report on 231 patients treated with the classic crush showed restenosis in over one fourth of the subjects, mainly at the SB ostium, at 8 months of follow-up31. In the first mini crush case series, comprising 52 bifurcation lesions in 45 patients, angiographic success was reported to be 100% but target lesion revascularisation was required in 12.2% patients at 8-month follow-up25. Limited data are available for nano and DK-nano crush, and the few studies available are consistent with high rates of technical success and good midterm results262728. Throughout the years, different crush technique iterations (classic crush, mini crush and DK crush) have been compared to one another and to other one- or two-stent strategies for the treatment of bifurcation lesions in the setting of randomised controlled trials, with mixed results. DK crush in particular has been most extensively studied. It was shown to be superior to classic crush23 and to provisional stenting32 in true bifurcations. In addition, it demonstrated superiority to culotte33 and, in the DKCRUSH-V randomised controlled trial, to provisional stenting for left main bifurcations21. A recent network meta-analysis including 21 randomised controlled trials and 5,711 subjects suggested that DK crush stenting may significantly reduce MACE rates compared to other techniques, particularly in the subgroup with SB lesions longer than 10 mm from the ostium9. Table 2 summarises relevant outcome data on the crush techniques.
INTRAVASCULAR IMAGING FOR BIFURCATION PCI
Intravascular imaging offers an advantage in terms of vascular characterisation with respect to coronary angiography. This translates in improved stent selection, as well as stent optimisation after deployment and ultimately in improved short- and long-term outcomes34. Both intravascular ultrasound (IVUS) and optical coherence tomography (OCT) can provide useful information. IVUS allows optimal stent sizing and provides help in detecting plaque-free landing zones35. It provides information on plaque distribution and characteristics (e.g., calcification) at the SB ostium and into the SB, which can eventually guide the decision to employ a two-stent technique upfront versus a provisional single stent. After stent deployment, IVUS can aid in optimising stent expansion35. While no randomised controlled trial evaluating IVUS-guided versus angiography-guided bifurcation PCI is currently available, registry data suggest that IVUS can reduce the incidence of MACE, including cardiac death, in the long term36. OCT provides similar data compared to IVUS in terms of plaque distribution and characteristics, vessel size, and landing zones37. The higher spatial resolution of OCT imaging may permit finer plaque composition analysis, and eventually precise evaluation of wire position after rewiring37. State-of-the-art OCT 3D reconstruction software allows precise ostium sizing and characterisation, assessment of bifurcation angle and wire position after SB rewiring, and detection of stent struts overhanging on the SB ostium38. All these features have the potential of improving bifurcation PCI results.
Conclusion
Since its introduction almost two decades ago, the crush technique for coronary bifurcation stenting has undergone a significant and constant evolution. In its later iterations, i.e., the DK crush technique, it appears to provide a significant advantage over other approaches to coronary bifurcation treatment in selected, complex cases. Novel outcome data on the latest iterations of the technique are eagerly awaited, as the nano crush iterations have the potential to overcome some shortcomings of the technique, i.e., the presence of multiple layers of struts in the proximal MB.
Acknowledgements
The authors would like to thank Victor Martinez and Emilie Min-Jen Yeh for their help with figure preparation.
Conflict of interest statement
A. Attallah reports receiving speakers’ bureau fees for Philips Volcano. R. Santiago has received speaker/proctoring honoraria from Boston Scientific, Abbott Vascular, and Teleflex. J. Hall has received honoraria from Boston Scientific. S. Bangalore reports being on the advisory board of Abbott Vascular, Biotronik, Pfizer, Reata, and Amgen. L. Azzalini has received honoraria from Teleflex, Abiomed, Asahi Intecc, Abbott Vascular, Philips, and Cardiovascular System, Inc. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

