
Abstract
Aims: The aim of this report was to examine the effect of underexpansion on stent thrombogenicity with an in vitro perfusion model.
Methods and results: Drug-eluting stent (DES) samples were partially underdeployed in silicone tubes and perfused with porcine blood containing 10% anticoagulant citrate dextrose solution for four minutes at a flow rate of 200 ml/min. Thrombus formation was evaluated and compared between the well-apposed and malapposed sections. The malapposed sections showed significantly more thrombus formation compared to the well-apposed sections (13.9 vs. 0.41 mm2, p<0.001).
Conclusions: Stent malapposition has a very direct impact on thrombus formation. Optimised stent implantation is required to minimise malapposition in DES and BVS to reduce thrombus formation.
Abbreviations
BMS: bare metal stent
DES: drug-eluting stent
GP: glycoprotein
ISA: incomplete stent apposition
LST: late stent thrombosis
PCI: percutaneous coronary intervention
VLST: very late stent thrombosis
Introduction
Pathological studies have revealed that malapposition and delayed re-endothelialisation are common findings in cases of late stent thrombosis (LST) after drug-eluting stent (DES) implantation1.
Malapposed struts perturb normal flow and are more likely to have delayed re-endothelialisation and remain uncovered as the distance to the vessel wall increases2. While pathological and in vivo observations suggest that incomplete stent apposition (ISA) can lead to stent thrombosis1,3, direct experimental evidence of this phenomenon remains scarce.
EFFECT OF STENT MALAPPOSITION ON THROMBUS FORMATION: EVIDENCE FROM PERFUSION MODELS
Kolandaivelu et al showed the impact of strut thickness on platelet adhesion using in vitro models. When perfused with porcine blood, a thin-strut bare metal stent (BMS) (81 μm) was shown to be less thrombogenic than an otherwise identical thick-strut (162 μm) stent4. Both were shown to attract clot rapidly when left underdeployed in the blood stream4. This previous in vitro perfusion experiment has shown that stent malapposition increases thrombogenicity in BMSs, but the same experiment did not show a significant effect with DESs.
Methods
Here, we examined the effect of underexpansion using an in vitro perfusion model. We analysed the difference in platelet adhesion and clot formation between well-apposed and malapposed stent sections of partially underdeployed DESs after four minutes of perfusion with porcine blood containing 10% anticoagulant citrate dextrose solution.
Results
Malapposed segments of the stents exhibited significantly more thrombus area on histology than well-apposed segments (13.9 vs. 0.41 mm2, p<0.001). Despite the formation of clot in the proximal malapposed segments of the stents, the distal well-apposed sections remained mostly free from clot adhesion on the surface of the strut (Figure 1).
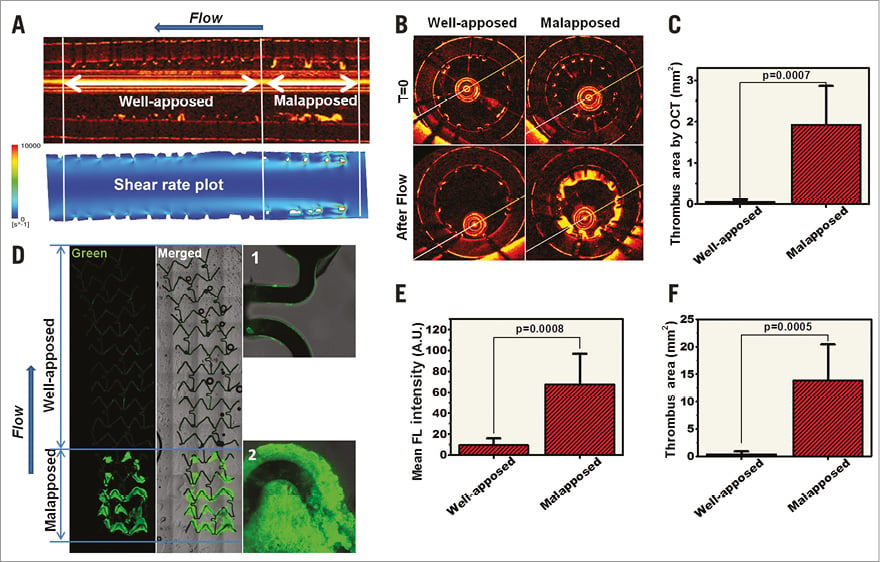
Figure 1. Impact of stent malapposition on thrombus formation. DES samples (3.0 mm XIENCE Prime™; Abbott Vascular, Santa Clara, CA, USA, and SYNERGY™; Boston Scientific, Marlborough, MA, USA) were each implanted in 3.0 mm silicone vessel models with their proximal segment partially underexpanded. After exposure to blood flow at a rate of 200 ml/min for four minutes, thrombus formation was evaluated in the well-apposed and malapposed stent segments for all the DES samples (total n=6). Representative longitudinal optical coherence tomography (OCT) (A) and confocal images (D) on a partially underexpanded XIENCE DES stent. Computational flow reconstruction of the shear flow profile in the malapposed stent (A, lower panel). B) Representative OCT cross-sections of the underexpanded and well-apposed segments of the stent before and after exposure to the blood flow. C) Average thrombus area in the well-apposed and malapposed segments were quantified from the OCT cross-sections in all samples. D) Antiplatelet immunofluorescence imaging on a longitudinal section of a stent. Mean fluorescent intensity (E) and thrombus area (F) quantified on immunofluorescence images of all samples. Magnification in panel D: 5× (left) and 20× (right, 1 and 2). Arrows in panels A and D indicate the direction of blood flow.
Discussion
Shear rate is the local gradient in velocity between adjacent blood flow streamlines; shear rate increases with disturbance in the flow and it is also a well-known modulator of platelet activation and thrombosis. High shear rate (>1,000 s–1) activates platelets in a dose-dependent manner through von Willebrand factor binding to glycoprotein (GP) Ib and GP IIb/IIIa receptors. Several experiments have shown the influence of flow and shear rate on clot formation in in vitro models and high shear rate is needed to reproduce the prothrombogenic environment required for thrombus formation in vivo. Normal human shear rate in large to medium-sized arteries usually remains below 1,000 s−1. However, during percutaneous coronary intervention (PCI), malapposed stent struts create front-facing obstacles that disrupt the blood flow stream and produce flow separation and large shear rates above 3,000 s–12.
Fortunately, stent thrombosis remains a rare phenomenon clinically, and not all stent malapposition causes thrombosis in patients adequately treated with efficient dual antiplatelet medications. While minor malapposition can self-correct with time, cases with significant persistent or acquired late malapposition raise more concern.
Incomplete stent apposition is considered a predictor of stent thrombosis and adverse outcomes3,5. The incidence of ISA has been reported in up to 77% of the cases of very late stent thrombosis (VLST) assessed by intravascular imaging3.
Limitations
For our experiment, we acknowledge that our in vitro experimental results might not be directly representative of thrombotic behaviour in the physiological environment in vivo. Our results should be seen more as an insight into the mechanistic effect of the local haemodynamic environment around unapposed segments on the thrombogenic cascade.
Conclusions
Stent thrombosis remains the most feared complication after PCI. Clinical and pathological evidence has previously shown that late ISA is a common finding in patients presenting with stent thrombosis. Experimental insights from in vitro models show the direct effect that a malapposed stent section can have on thrombus formation compared to well-apposed segments.
| Impact on daily practice The effect of malapposition on stent thrombosis still has important implications for PCI procedures and stent optimisation. Clinical studies using DESs have recently supported optimisation with high-pressure balloons to reduce underexpansion and malapposition. Better stent apposition does translate to better outcome, and experiments suggest that optimal apposition may improve local haemodynamic environment and also reduce potential thrombogenic stimuli. |
Conflict of interest statement
R. Virmani has speaking engagements with Merck, receives honoraria from Abbott Vascular, Boston Scientific, Lutonix, Medtronic, and Terumo Corporation, and is a consultant for 480 Biomedical, Abbott Vascular, Medtronic, and W.L. Gore. N. Foin holds a salaried appointment from Philips. M. Joner is a consultant for Biotronik, is in receipt of a grant from the European Commission, and has received speaking honoraria from Abbott Vascular, Biotronik, Medtronic, Boston Scientific, and OrbusNeich. The other authors have no conflicts of interest to declare.

