Abstract
Aims: The Sideguard® stent (Cappella Medical Devices Ltd, Galway, Ireland), is a novel nitinol self-expanding dedicated bifurcation stent that flares proximally at the ostium of the side branch (SB) into a trumpet shape thereby achieving full ostial coverage. The aim of this study is to report the utility and limitations of this stent in patients undergoing treatment to bifurcation coronary lesions in a real-world setting.
Methods and results: We prospectively identified 20 successive patients admitted over a 6-month period in whom there was significant SB disease and who were suitable for a bifurcation procedure. The Sideguard® stent was successfully used in all 20 cases including several that would have been technically difficult using conventional bifurcation techniques. We highlight use of this system using five illustrative cases that illustrate its utility and limitations in the treatment of bifurcation lesions.
Conclusions: The Sideguard® stent can be used to treat complex bifurcation lesions in a straight forward manner and is not subject to the limitations associated with conventional bifurcation PCI techniques including jailing of the SB ostium and inability to fully cover/scaffold the ostium of the SB.
Introduction
Bifurcation lesions are commonplace in contemporary percutaneous coronary interventional practice and account for up to about 15-20% of PCI procedures performed1. PCI for bifurcation disease is considered technically challenging and has been associated with both lower procedural success rates and higher adverse events rates than observed in non-bifurcation lesions2,3. The optimal treatment strategy for bifurcation lesions is unclear, in the recent British Bifurcation Coronary Study (BBC-1) a systematic 2-stent technique for the treatment of bifurcations lesions results in a two-fold increased risk of death, myocardial infarction and target-vessel failure compared to a provisional single stent strategy4; although in other studies –such as NORDIC1 and CACTUS5– have shown no differences in MACE outcomes between systematic 2-stent strategies and a single provisional stent strategy.
Irrespective of the bifurcation stent strategies undertaken, there are a number of technical limitations to current bifurcation stent strategies such as maintaining access to the side branch (SB) throughout the procedure; jailing of the SB ostium by the main branch stent struts resulting in difficulty in either rewiring the SB or passing a further balloon or stent into the SB and the inability to fully cover and scaffold the ostium of the SB2,6. Differences in diameter between the SB and main branch (MB) or acute angulations of the SB relative to the MB further increase the complexity of bifurcation procedures.
Consequently, a number of dedicated bifurcation stents have been developed to overcome many of the technical difficulties associated with PCI of bifurcation lesions6. Types of dedicated bifurcation stents that exist include preformed stents with side ports to facilitate access to the SB following treatment of the MB or stents designed to treat the SB vessel first2,6. An example of the latter is the Sideguard® stent (Cappella Medical Devices Ltd, Galway, Ireland), a novel nitinol self-expanding stent that flares proximally at the ostium of the SB into a trumpet shape thereby achieving full ostial coverage. Here we report use of this novel dedicated bifurcation system in 20 consecutive patients undergoing treatment to bifurcation coronary lesions in a real-world setting and discuss the clinical utility and limitations of this system in the treatment of such lesions.
Methods
CAPPELLA SIDEGUARD® STENT
The Sideguard® stent (Cappella Medical Devices Ltd, Galway, Ireland), is a CE approved self-expanding nitinol stent indicated for bifurcation angles from 45º to 135º before wiring that flares proximally at the ostium of the SB into a trumpet shape to achieve full ostial coverage unlike conventional stents (Figure 1A). The product is comprised of two components: The implantable coronary side branch stent and a low profile delivery system. The nitinol self-expanding stent has a section which functions to anchor the device in the SB, and a proximal flared section that is intended to cover the side branch ostium. The device has five radiopaque markers, three radiopaque proximal markers to aid accurate positioning of the stent at the SB and two distal radiopaque markers that allow angiographic visibility post-implantation. The stent is available in diameters of between 2.25-3.25 mm and a length of 10 mm. The stent is deployed using a nominal pressure balloon, which helps tear a protective sheath that keeps the Sideguard in place until deployment (Figure 1B). Once released, the Sideguard self-expands into place (Figure 1C). This novel means of stent deployment is different to previous self-expanding stents that rely on a deployment sheath that covers the stent and, when retracted allows the stent to self-expand7. The delivery system and the guidewire can then be removed from the SB, and further stents can be delivered distally if required. If the MB is also to be treated, a conventional stent can be deployed as per usual practice.
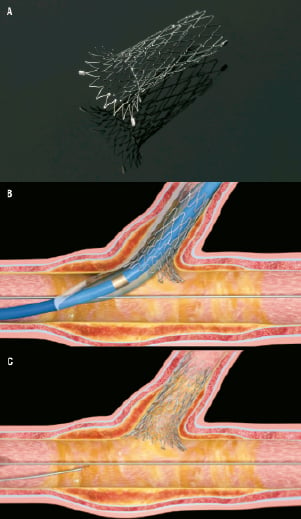
Figure 1. (A) Sideguard® stent with trumpet shaped end to enable full ostial coverage. (B) Deployment of Sideguard® stent. Inflation of the balloon tears the protective sheath that enables self-expansion of the Sideguard® stent. (C) Final result.
PROCEDURAL TECHNIQUES
We prospectively studied 20 successive patients admitted to Manchester Heart Centre (Manchester, UK) over a 6-month period (March 2010 until September 2010) in whom there was significant SB disease and who were suitable for a bifurcation procedure. All elective patients were referred for PCI based on clinical symptoms or inducible ischaemia documented by noninvasive stress testing. All patients were pre-treated with aspirin (300 mg) and clopidogrel (300 mg-600 mg) and 75 mg thereafter for at least one year. In all cases in which bifurcation procedures where performed using the Sideguard stent, both the MB and SB were pre-dilated with either compliant or non-compliant balloons and a standard final kissing balloon was performed although the manufacturer does not state this to be mandatory.
CLINICAL ENDPOINTS
Clinical endpoints studied included periprocedural and 6-month all-cause mortality and major adverse cardiac events (MACE) defined as the composite endpoint of all-cause mortality, stroke, myocardial infarction (MI), stent thrombosis and target lesion (TLR) /vessel revascularisation (TVR). TVR /TLR was defined as a clinically driven or ischaemia tested driven revascularisation of the index vessel/lesion. Repeat revascularisation procedures, episodes of MI and complications were collected prospectively from the Manchester Heart Centre PCI database in which procedural, clinical and demographic data are entered for each patient undergoing PCI prospectively and retrospectively. Data quality entered into this database is crosschecked and validated by an independent Clinical Information Assistant using the PCI procedural reports generated by the operator and information obtained from the medical notes. Clinical follow-up was completed in all patients at six months post procedurally.
QUANTITATIVE CORONARY ANGIOGRAPHY (QCA)
Angiographic analysis of bifurcation lesions using standard QCA packages that are designed for the analysis of single lesions in ‘‘straight’’ vessels is limited due to the challenge in defining the true reference vessel size of both the parent vessel and its side branch. In the bifurcated coronary anatomy, the reference diameter of the main vessel ‘‘steps down’’ from proximal to distal relative to the side branch whilst standard QCA algorithms are designed to detect vessel contours assuming minimal vessel tapering. Consequently, the reference vessel dimensions are inherently inaccurate when applied to bifurcation lesions8. The European Bifurcation Club has recently published a consensus statement outlining a standard approach for the analysis and reporting of the angiographic results of the bifurcation lesion using dedicated bifurcation systems8. QCA analysis was performed in accordance with these recommendations in a core laboratory by the co-author AL using Medis software (Qangio XA, Medis, Leiden, The Netherlands) as per previously published protocols1.
RESULTS
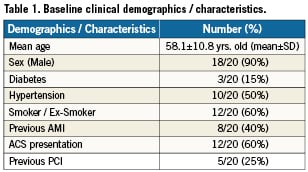
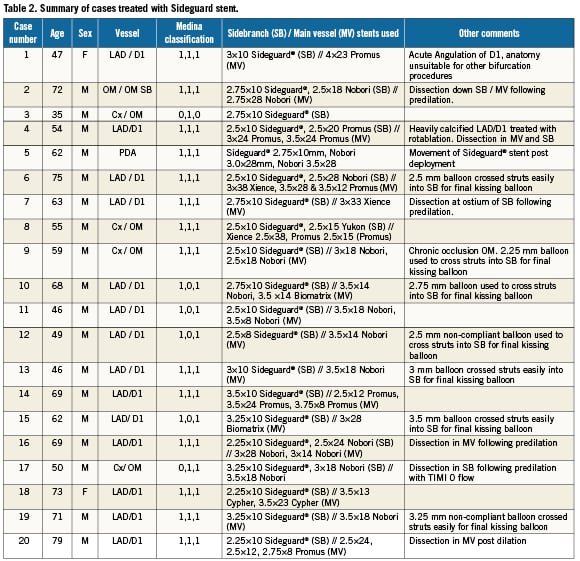
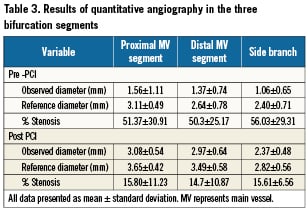
A total of 815 PCI procedures involving 977 lesions were performed over a 6-month period at the Manchester Heart Centre (March-September 2010) of which 158 lesions were bifurcation lesions (16.1%). Of these lesions, 123 were treated with a single stent strategy (77.8%) whilst 35 lesions where treated with a two-stent strategy (22.2%). A total of 20 cases where performed over a 6-month period using the Sideguard® stent, of which 19 were true bifurcation lesions (95%) and 14/20 (70%) were classified as 1.1.1 according to the medina classification. Eight of these cases were elective PCI admissions and the remaining 12 patients underwent PCI following admission with an acute coronary syndrome. Mean age of the patients was 58.1±10.8 years old (mean ± SD) and 18 of the 20 patients were male. Patient demographics are presented in Table 1. A summary of the 20 cases performed is presented in Table 2. All cases were performed with a balanced middle weight (BMW) or choice floppy wires and the SB was easily rewired following deployment of the MB stent in all cases with a single wire to enable final kissing balloons. Final kissing balloons were performed in all cases that involved a true bifurcation (19/20) and delivery of compliant (up to 3.5 mm diameter) or non-compliant balloons (up to 3.25 mm diameter) into the SB was performed with relative ease during the final kissing balloon procedure in most cases (16/19 cases) without the need to perform pre-dilation of the stent struts with a smaller balloon initially.
QCA analysis was performed for the 20 cases pre- and post- procedurally, this is summarised in Table 3. The side branch angulation was >60° in 13/20 cases (65%) and there was at least moderate calcification in the MV or SB in 7/20 cases (35%). No periprocedural complications were recorded in the 20 cases performed although in one case outlined below (case 5), significant stent displacement of the Sideguard stent post-deployment occurred. At six month follow-up, a MACE event rate of 5% was recorded, in which a patient re-presented with an ACS five months following the index procedure and underwent TLR with a drug-eluting balloon as outlined below in case 3. The remaining 19 patients remain MACE free at 6-month follow-up.
Five representative cases from this series are presented below that highlight the utility as well as the limitations of the Sideguard system in the treatment of complex bifurcation procedures.
CASE 1
The first case illustrates the utility of the Sideguard® system in treating acutely angulated bifurcation lesions that may be difficult to treat using conventional bifurcation techniques. In this case, a 47-year-old female was admitted following an acute coronary syndrome (ACS). Cardiac catheterisation demonstrated a segment of severe disease involving the proximal LAD and a large diagonal at 90 degrees to the main vessel, with further disease within the LAD more distally (Medina 1,1,1); Figure 2A. Both lesions were pre-dilated and a 3×10 mm Sideguard® stent was deployed in the diagonal branch (Figure 2B). A Promus 4×23 mm stent was deployed in the LAD crossing the diagonal vessel (Figure 2C) and the diagonal vessel was re-crossed with a BMW wire and the SB ostium was predilated with successively larger balloons from 1.25 mm upwards until a 3 mm balloon could be delivered and final kissing balloon performed. The final result is illustrated in Figure 2D.
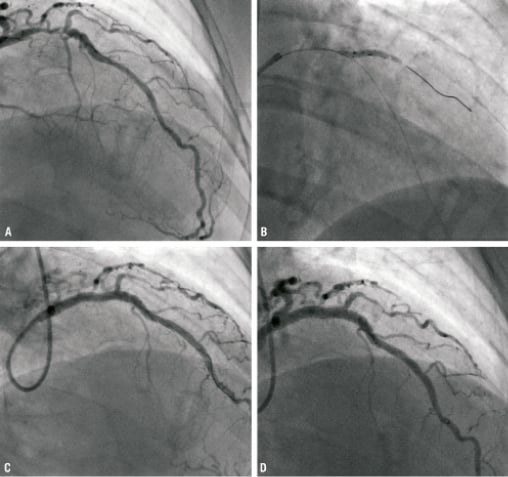
Figure 2. (A) Initial angiogram illustrating bifurcation lesion involving LAD and D1 (Medina 1.1.1). (B) Sideguard® stent deployment in SB (C) Post deployment of Promus 4×23 mm stent in LAD. (D) Final result. (E) Angiographic follow-up at six weeks.
CASE 2
The second case illustrates the utility of the Sideguard® system in maintaining access to the SB during the bifurcation procedures that are complicated by coronary dissections in the side branch. A 72-year-old male was admitted with an acute coronary syndrome with lateral ECG changes. Cardiac catheterisation (Figure 3A) demonstrated a segment of severe disease involving a large obtuse marginal (OM) and an obtuse marginal side branch (Medina 1,1,1). Both lesions were predilated but a significant dissection developed within the OM SB (Figure 3B) with subsequent development of TIMI 1 flow and lateral ECG changes. A 2.75×10 mm Sideguard® stent was deployed in the OM side branch (Figure 3C), and a second 2.5×18 mm Nobori stent was deployed more distally. A Nobori 2.75×28 mm stent was positioned in the OM (Figure 3D) and deployed at high pressure. Final kissing balloons were performed with the final result presented in Figure 3E. The patient remains well at six month follow-up.
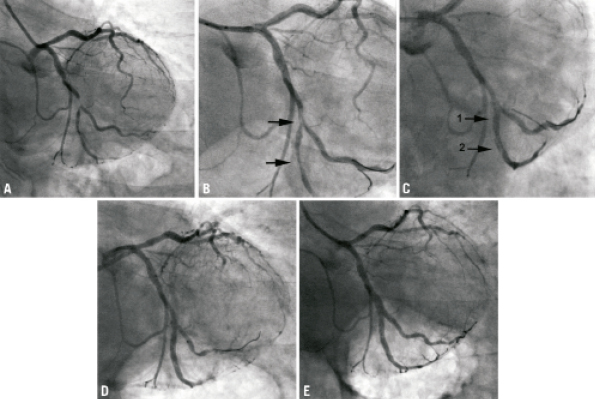
Figure 3. (A) Initial angiogram illustrating bifurcation lesion involving OM and OM side branch (Medina 1,1,1). (B) Following predilation with arrows illustrating dissection in OM side branch. (C) Following Sideguard® stent deployment (arrow 1) a dissection can be seen to extend distal to the stent (arrow 2). (D) 2.5×18 mm Nobori stent positioned in OM side branch overlapping Sideguard® stent and covering distal dissection. (E) Positioning of main vessel stent (F) Final result.
CASE 3
The third case illustrates how self-expanding stents such as the Sideguard® system can overcome significant intimal hyperplasia to maintain the luminal area of the SB by an increase in stent area over time. A 35-year-old diabetic male presented with a NSTEMI and cardiac catheterisation demonstrated a circumflex (Cx) lesion next to a large OM (Figure 4A; Medina 0,1,0). The Cx lesion was predilated with a 2.5 mm balloon and a 2.75×10 mm Sideguard® stent was deployed with the final result illustrated in Figure 4B. OCT was performed, and the stent was well opposed with a mean stent area of 5.47 mm2 (Figure 4C) and full coverage of ostium without disturbing the healthy adjacent large OM vessel. The patient was readmitted eight weeks later for further elective PCI, and the stent was revealed to be widely patent (Figure 4D). Repeat OCT examination of the Cx (Figure 4E) illustrated significant intimal hyperplasia although the stent area had increased significantly to 6.56 mm2 and the luminal area was 4.21 mm2. The patient was re-admitted five months following the index procedure with a further ACS with lateral T wave inversion on the ECG and repeat coronary angiography revealed significant in-stent restenosis (Figure 4F) that was treated with a drug-eluting balloon (Figure 4G). The patient remains well at three month follow-up.
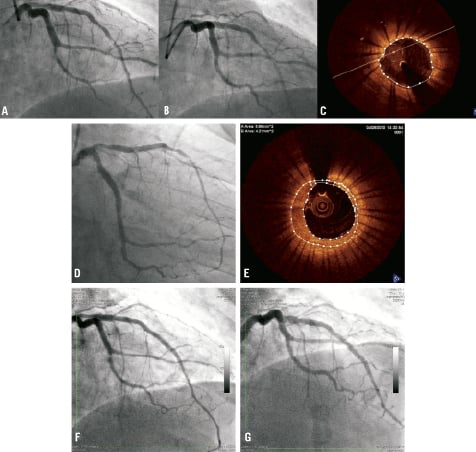
Figure 4. (A) Initial angiogram illustrating lesion in circumflex next to a large OM vessel (Medina 0,1,0). (B) Angiogram post deployment of Sideguard® stent. (C) OCT appearance of stented segment of the Cx at the end of the case. Angiographic (D) and OCT (E) appearances of circumflex vessel following re-admission eight weeks later for elective PCI to LAD. (F) appearance of circumflex following admission with acute coronary syndrome and (G) post-drug-eluting balloon treatment.
CASE 4
This illustrative case demonstrates the deliverability of the Sideguard® stent in heavily calcified vessels, and how it may act to protect the patency of the SB, particularly when significant dissections occur in the main vessel. A 65-year-old male was admitted for elective PCI to a heavily calcified LAD / D1 bifurcation lesion (Figure 5A; Medina 1,0,1). Rotablation was performed with a 1.25 mm burr successively down the LAD and D1 and subsequent balloon predilation was performed in both vessels. A 2.5×10 mm Sideguard® stent was deployed in the D1 SB (Figure 5B). Further dilation of the main branch was required with a 3.5 mm non-compliant balloon and a spiral dissection extending down the LAD was visualised (Figure 5C). A 3×24 mm Promus was deployed distally and overlapping this was a 3.5×24 mm Promus stent deployed more proximally. A dissection was noted just distal to the Sideguard® stent in the SB (Figure 5D) therefore the D1 was rewired with a BMW wire and a 2.5×20 Promus was delivered through the Sideguard® stent without resistance and deployed more distally to cover the dissection. The final result is illustrated in Figure 5E.
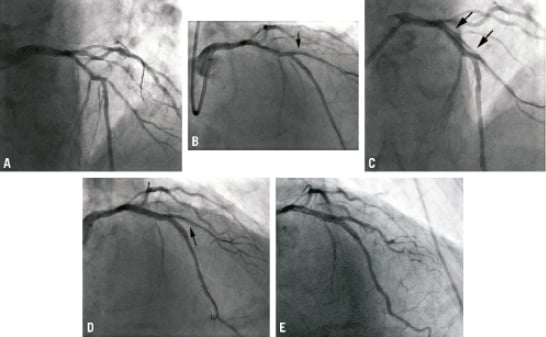
Figure 5. (A) Initial angiogram illustrating heavily calcified LAD / D1 bifurcation lesion (Medina 1,0,1). (B) Post Sideguard® stent deployment. (C) Spiral dissection visualised down LAD (illustrated by arrows) following further balloon predilation. (D) Further dissection (shown by arrow) seen in D1 distal to Sideguard® stent position. (E) Final result.
CASE 5
This case demonstrates the importance of allowing the stent delivery balloon to fully deflate before it is removed post-deployment of the Sideguard® stent, since the torn protective sheath may lead to stent displacement. A 62-year-old male was admitted with an inferior STEMI for primary PCI. Diagnostic cardiac catheterisation illustrated an occluded mid RCA (Figure 6A). Extensive thrombectomy was performed and both the PDA and PLV branches were predilated. A Sideguard 2.75×10 mm stent was positioned in the ostium of the PDA and deployed successfully (Figures 6B and 6C). Following stent deployment, premature retrieval of the balloon resulted in migration of the Sideguard stent into the distal RCA just proximal to the bifurcation of the PDA/PLV (Figures 6D and 6E). A Nobori 3.0×28 mm stent was passed through the Sideguard stent now positioned in the distal RCA and deployed across it into the PDA branch (Figure 6F). Overlapping this, a Nobori 3.5×28 mm stent was deployed more proximally. The final result is illustrated in Figure 6G.
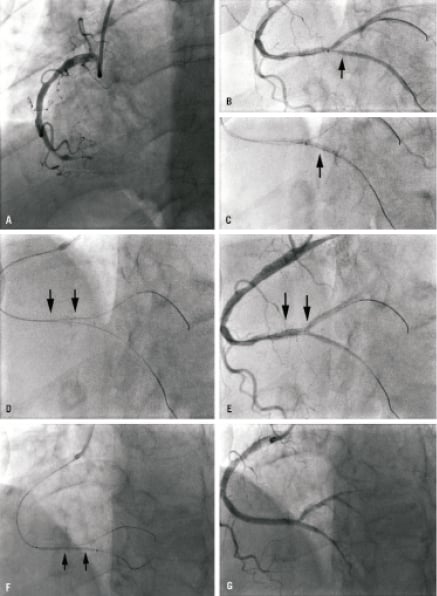
Figure 6. (A) Initial angiogram on presentation with inferior STEMI. (B) Positioning of Sideguard® stent in PDA and deployment (C). (D and E) Migration of Sideguard® stent into distal RCA. Arrows illustrate proximal and distal edges of stent respectively. (F) Nobori 3.0×28 mm stent passed through the Sideguard® stent that had migrated into the distal RCA and deployed across it into the PDA branch. Edges of Sideguard® stent highlighted by arrows. (G) Final result.
Discussion
In this prospective case series, we report the successful use of the Sideguard® stent in 20 cases of which 19 involved bifurcation lesions and the remaining case involved isolated side branch (SB) ostial disease. In all patients, delivery of the Sideguard stent was successful despite passage through dissections caused by predilation of the lesions in four cases (cases 3,7,16 and 17), acute angulation of the SB of at least 90° (case 1), chronically occluded SB (case 9) and heavily calcified SB necessitating rotablation (case 4). We have found that treatment of bifurcation lesions using the Sideguard stent to be straight forward and not associated with jailing/loss of the SB resulting in difficulty in either rewiring the SB or passing a further balloon or stent into the SB which is occasionally encountered when treating bifurcation lesions with conventional PCI techniques. Re-wiring of the SB was easy in all cases since the wire only had to pass through one layer of struts (that of the main vessel) and delivery of balloons up to 3.5 mm in diameter was performed with relative ease without the need to perform predilation of the stent struts initially with a smaller balloon in most cases. This is in contrast to the situation encountered not infrequently in bifurcation lesions treated with conventional 2-stent strategies, where difficulties are encountered with either rewiring of the SB or delivery of a balloon to the SB to enable a final kiss, due to having more than one layer of stent to pass through or an incompletely expanded SB ostium. For example, in the BBC-1 study, there was a 15% failure rate in kissing balloons in the 2-stent-strategy arm either due to failure to rewire the vessel or balloon delivery4.
Differences in diameter between the SB and main vessel, or acute angulations of the SB relative to the MB, further increase the complexity of bifurcation procedures treated through conventional means and limit the applicability of some techniques such as culotte. For example, in our series, there was a significant size mismatch of at least 1 mm between the main vessel and the SB in one fifth of cases. Case 1 (Medina 1,1,1) illustrates the limitations of conventional techniques in the treatment of complex bifurcation anatomies with adverse characteristics since the acute angulation of the SB and the mismatch in size would make this case difficult to treat by conventional 2-stent approaches. In this case, using a provisional T-stent strategy may result in loss of the SB following deployment of the main vessel stent first since the acute angulation makes rewiring and delivering another stent through the main vessel stent struts into the SB problematic. Similarly with the crush technique, re-entry of SB through two layers of struts and delivery of a balloon for final kissing balloon would be particularly difficult; equally the Culotte technique would not be suitable due to acute angle and miss-match between SB and main vessel size. The treatment of this complex bifurcation was rendered simple and straightforward using the Sideguard® stent with none of the limitations faced by conventional 2-stent strategies.
Previous studies have shown that treatment of bifurcation lesions is associated with a high rate of restenosis at the SB ostium, irrespective of whether a provisional single stent strategy or two stent bifurcation strategy is used: for example, 6-month SB restenosis rates of 21.8% have been recorded in bifurcation lesions treated with a 2-stent strategy and 14.2% treated with a single-stent strategy using sirolimus eluting stents9. Similarly, in the more recent randomised trial of Ferenc et al, angiographic re-stenosis of the SB at nine months was documented in 23% of provisional single-stent strategies, and 27.7% in systematic 2-stent strategies10.
The high restenosis rate observed at the SB ostium in 2-stent strategies may be a function of stent under-expansion and inadequate SB stent coverage11,12. Costa et al have reported that following crush stenting of bifurcation lesions, incomplete apposition of the three layers of main vessel and SB stent struts was seen in 60% of bifurcation lesions on IVUS examination, and that the majority of SB stents showed under-expansion, particularly at the SB ostium12. Similarly, IVUS examination of bifurcation lesions treated with T-stenting, revealed significant stent under-expansion in the SB particularly in the SB ostium13. Ostial SB stent under-expansion may be an important mechanism of restenosis with drug-eluting stents because even a small amount of intimal hyperplasia superimposed on significant stent under-expansion can result in restenosis12.
Inadequate SB ostial coverage following SB stenting may also account for the high restenosis rates observed in SB. IVUS studies of restenosis in stented SB treated with sirolimus eluting stents have shown incomplete ostial stent strut coverage with evidence of focal neointimal hyperplasia at the ostium. Studies have shown incomplete coverage of the SB in 8% of T-stent cases of up to 3 mm in length13.
The Sideguard® stent design addresses these two main mechanisms thought to account for the high rates of SB restenosis encountered in clinical practice: stent under-expansion and inadequate SB stent coverage. Self-expansion of the Sideguard stent overcomes the problem of under-expansion encountered in the SB12,13. Doi et al14 have recently reported serial IVUS examination (baseline and 6-month follow-up) in 11 patients who were treated with Sideguard stents as part of the Sideguard I First-In-Man study, where the authors did not report any significant restenosis at six months on IVUS examination, despite significant intimal hyperplasia due to the self-expansion of the Sideguard stent resulting in significant increases in stent area at the SB carina, thereby compensating for any intimal hyperplasia resulting in no net change in luminal area. Similarly in the Stent COmparative REStenosis (SCORES) trial, serial IVUS analyses in this trial showed that stent area increased by 33% at six months in the self-expandable group, resulting in no change in lumen area despite the greater amount of intimal hyperplasia (3.1±2.0 vs 1.7±1.5 mm2, p <0.01) compared to the balloon expandable stent group15. Similarly, in case three in which we performed OCT examination at baseline and at eight weeks, we observed a significant increase in stent area and, despite significant intimal hyperplasia, the luminal area remained >4 mm2, although on longer-term follow-up in which further stent expansion would be minimal, a continued intimal hyperplastic response might result in clinically apparent re-stenosis such as observed in case 3.
Previous studies have observed worse restenosis rates in SBs treated with BMS compared to DES16,17. Although the Sideguard® stent is a BMS stent, it has many advantages compared to conventional stents used for the treatment of SD such as self-expansion and increased ostial coverage, therefore the restenosis outcomes may be potentially better than conventional BMS. Furthermore, in a recent study, five different DES (Cypher, Cypher Select, Endeavor, Taxus Express, and Taxus Liberté) deployed using kissing post-dilatation protocols in a bench-top bifurcation model demonstrated that DES over-inflation reduced the ratio of potential “metal to artery” thereby reducing drug application area in these over-expanded segments in the SB, hence limiting the antiproliferative effect of the DES18. In addition, using micro focus x-ray CT and scanning electron microscopy, the authors demonstrated significant coating damage on the ostial struts, thereby further reducing drug delivery to the SB ostium and thus increasing the risk of restenosis. Consequently, the efficacy of conventional DES used in the treatment of SB are reduced due to decreased ostial coverage, due to which antiproliferative drugs may not reach the ostial wall and significant coating damage may occur on the ostial struts further limiting drug efficacy.
Long-term efficacy data related to the use of the Sideguard® stent for treatment of bifurcation lesions is lacking, future larger registry studies are planned to provide further data relating to efficacy and MACE outcomes associated with the use of the Sideguard stent (The Sideguard Coronary Side branch Registry). Nevertheless, we have found it both easy-to-use and efficacious in the treatment of bifurcation lesions. There are a number of points that must be borne in mind when using the Sideguard stent. Firstly, the Sideguard® bifurcation system is not suitable for bifurcation lesions with angles of <40° and, due to their 10 mm length, they are only able to treat the ostium. Longer SB lesions would require the use of further overlapping stents. Secondly, the inflation of the balloon during stent deployment results in the tearing of a protective sheath that allows the Sideguard to self-expand. If the balloon is not fully deflated once the stent is deployed, there is a risk that the torn protective sheath may result in displacement of the Sideguard stent, as was the observed in case 5. Finally, longer-term efficacy data is awaited.
In conclusion, we have found that treatment of bifurcation lesions using the Sideguard stent to be straight forward and not subject to the technical limitations associated with conventional PCI techniques and the Sideguard® stent design overcomes the mechanisms thought to underlie the high restenosis rates observed at the SB ostium in the 2-stent strategies, namely stent under-expansion and inadequate SB stent coverage.
Conflict of interest statement
F. Fath-Ordoubadi has received fees for consulting from Cappella Medical Devices Ltd, Galway, Ireland. All other authors report no conflicts of interest.

