
Abstract
Aims: We aimed to assess the safety and efficacy of the DynamX Novolimus-Eluting Coronary Bioadaptor System, a novel device that initially acts as a second-generation drug-eluting stent, but after six months frees the vessel through uncaging elements.
Methods and results: This multicentre study enrolled 50 patients with single de novo lesions. In-device acute lumen gain was 1.61±0.34 mm, and device and procedure success was 100%. Up to 12 months, two target lesion failures occurred: both were cardiac deaths (day 255 and day 267 post procedure). No definite or probable device thrombosis was observed. Mean late lumen loss was 0.12±0.18 mm in-device and 0.11±0.16 mm in-segment. Per intravascular ultrasound, the mean device area and mean vessel area increased significantly by 5% and 3%, respectively, while the mean lumen area was maintained. Stationary optical coherence tomography in seven patients demonstrated restoration of cyclic pulsatility, with an approximate lumen area variance of 11% between systole and diastole.
Conclusions: The DynamX bioadaptor showed drug-eluting stent-like acute performance and safety and efficacy up to one year. Positive remodelling with an increase of vessel and device area while maintaining the mean lumen area was demonstrated. Long-term follow-up and randomised trials are required to assess the benefit of this device on events beyond one year.
Introduction
Improvements in device technology have reduced early event rates after drug-eluting stent (DES) implantation but, beyond one year, a persistent 2-4% annual incidence of major adverse events or target lesion failure (TLF) without plateau is observed1,2. Device-related factors contributing to these events are that conventional DES “cage” the coronary artery, causing mechanical distortion, preventing positive adaptive remodelling and pulsatility, as well as serving as a nidus for chronic inflammation and strut fractures2. Although bioresorbable scaffolds attempted to address these issues by providing structural support to the vessel early on, followed by resorption, randomised trials showed that, prior to their resorption, they were less safe and effective than contemporary DES3,4.
The DynamX™ Novolimus-Eluting Coronary Bioadaptor System (Elixir Medical Corporation, Milpitas, CA, USA) is a novel device with a series of unique characteristics that gained CE mark in September 2019. It is a thin (71 µm) cobalt-chromium platform that offers a fundamental innovation in stent design, incorporating uncaging elements in the sinusoidal rings. The thin polymer coating on the uncaging elements resorbs over six months, allowing the device to accommodate vessel expansion. In this context, the DynamX bioadaptor combines the acute performance of contemporary DES with the unique benefit of arterial “uncaging” to permit a return towards cyclic pulsatility and compensatory positive adaptive remodelling, which may result in fewer clinical events beyond one year. We report the first clinical experience from the DynamX study.
Methods
STUDY DESIGN
DynamX is a prospective, single-arm feasibility study to assess the safety and performance of the novolimus-eluting DynamX Bioadaptor System. Patients were enrolled at six centres in Belgium and Italy from November 2017 to September 2018. Follow-up assessments were scheduled at 30 days, 6, 12, 24 and 36 months, and angiographic and intravascular ultrasound (IVUS) follow-up at 9-12 months. Optical coherence tomography (OCT) was performed at two centres.
The full list of inclusion and exclusion criteria is provided in Supplementary Table 1 and at ClinicalTrials.gov (NCT03429894). In brief, patients with a single de novo target lesion were included. The concomitant treatment of a single, non-target lesion in a separate major epicardial vessel prior to target lesion treatment was allowed.
The study was conducted according to the Declaration of Helsinki, ISO14155 and local regulations, and was approved by the respective ethics committees. Each patient provided written informed consent. Monitoring included source document verification, and an independent clinical events committee adjudicated all events. A list of study centres and study committees is provided in Supplementary Table 2.
STUDY DEVICE
The DynamX bioadaptor is composed of 71 µm cobalt-chromium sinusoidal rings connected to each other axially by three S-links which remain intact after uncaging. Each ring contains three uncaging elements that are positioned at equal distance in low-stress regions of struts oriented in a helical configuration along the length of the bioadaptor (Figure 1). The uncaging elements consist of three separable junctions per ring held together by a 6 µm polymer coating that is resorbed over six months, allowing uncaging of the vessel and adaptive remodelling (Supplementary Figure 1). The bioadaptor is circumferentially coated with a thin conformal, bi-layer biodegradable polymer, similar to that used on the DESolve scaffold and DESyne® BD DES (Elixir Medical)5,6. The inner bioresorbable coating is poly-l-lactide acid-based and the outer coating is poly-lactic-co-glycolic acid-based and releases the sirolimus metabolite novolimus over three months. The study device was available in diameters of 2.5, 3.0 and 3.5 mm and lengths of 14, 18 and 28 mm.
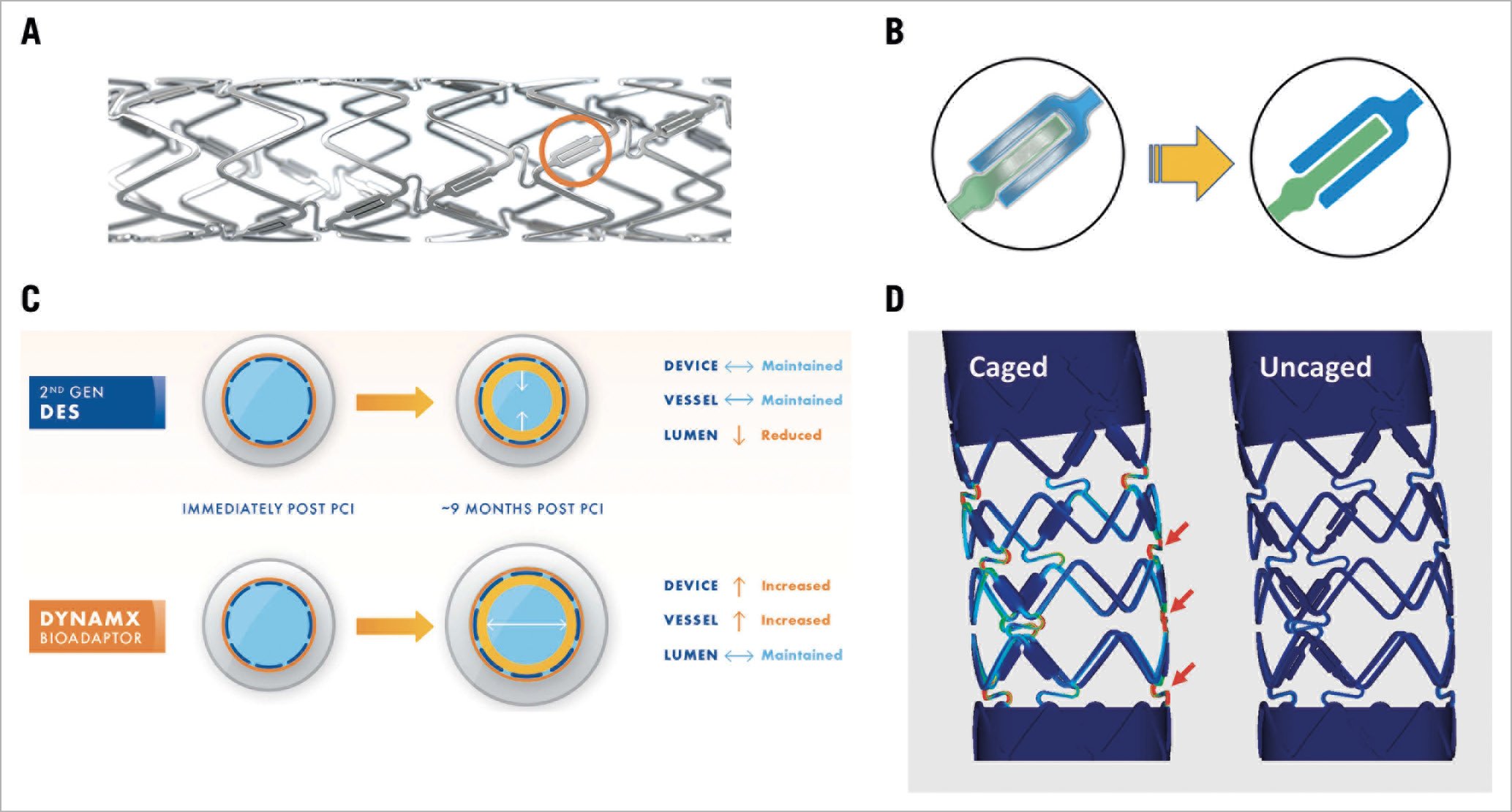
Figure 1. DynamX bioadaptor. A) DynamX bioadaptor with three uncaging elements (orange circle) per sinusoidal device ring. B) The uncaging elements disengage after polymer resorption allowing positive adaptive remodelling. Left: the two components of the uncaging element tightly held together by the conformal biodegradable polymer coating. Right: following polymer degradation, the elements disengage thus allowing the artery to uncage circumferentially. C) The schematic illustration of Glagov remodelling9 shows that the uncaging allows the vessel to enlarge to accommodate neointimal formation and disease progression to maintain lumen area. Blue circles reflect the device, grey circles the vessel, and yellow circles the neointimal hyperplasia. D) Reduction in tensile stress in caged versus uncaged DynamX bioadaptors loaded as in the Ormiston test21. The colours indicate von Mises stress in the bioadaptor segment, with cooler colours (blue) indicating lower stress, and hotter colours (red) indicating higher stress (data available at Elixir Medical).
PROCEDURE
Predilatation of the target lesion was recommended with a balloon size approximately 0.25-0.5 mm smaller than the mean vessel reference diameter. A residual stenosis of <35%, Thrombolysis In Myocardial Infarction (TIMI) flow of ≥2 and no greater than a Grade B dissection able to be covered by a single device were prerequisites for implantation of the bioadaptor that is implanted in a similar way to a traditional DES. All patients received a loading dose of acetylsalicylic acid and a P2Y12 inhibitor pre-procedure followed by dual antiplatelet therapy for 12 months; statin therapy was recommended.
IMAGING ANALYSIS
All imaging analyses were performed at an independent core laboratory (Cardiovascular Research Center [CRC], São Paulo, Brazil) by experienced operators blinded to procedural data and clinical outcomes. Further information is provided in Supplementary Table 3 and Supplementary Table 4.
ENDPOINTS
The primary safety endpoint was target lesion failure (TLF) at six months, a composite of cardiac death, target vessel myocardial infarction (TV-MI), and clinically driven target lesion revascularisation (CD-TLR). The primary imaging/efficacy endpoint was change in mean in-device and mean lumen area from post procedure to 9-12 months by IVUS. Secondary endpoints were TLF at other time points, target vessel failure (TVF), MI, TLR, target vessel revascularisation (TVR), and device thrombosis. Device success was defined as successful delivery of the bioadaptor to the target lesion and a final residual stenosis <30% by QCA (by visual estimation if QCA was unavailable). Procedure success was defined as device success without the occurrence of in-hospital TLF. The endpoint of TVF comprised a composite of cardiac death, TV-MI, and clinically driven TVR. Cardiac death and device thrombosis were defined according to the Academic Research Consortium criteria7. Per protocol, MI was defined as enzyme elevation of two times the upper normal limit of CK with elevation of CK-MB.
STATISTICAL ANALYSIS
This study was designed to confirm the feasibility, performance, and safety of the DynamX bioadaptor and to generate hypotheses for future studies. The sample size was not calculated based on an endpoint hypothesis, but is in line with the current ESC-EAPCI Task Force report that recommends a sample size of 50-150 patients for feasibility studies8. A maximum drop-out rate of 10% for follow-up visits and 20% for angiographic assessments was expected.
Descriptive statistics of the intention-to-treat population include continuous variables expressed as mean±standard deviation and median and interquartile range as applicable, and categorical variables expressed as frequencies and percentages of the total; 95% confidence intervals (CI) were calculated as applicable. Pairwise comparisons between post procedure and follow-up were performed by the Wilcoxon signed-rank test. OCT results are presented using the generalised linear mixed model with gamma distribution and random effects by lesion to adjust for cluster distribution of continuous variables at the strut and cross-section levels. In stationary OCT analysis, vessel pulsatility was assessed with the 95% predictive interval relative to the mean for the changes observed in the lumen and bioadaptor areas. All calculations are based on the data available. The analysis was performed by an independent statistician at CRC using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
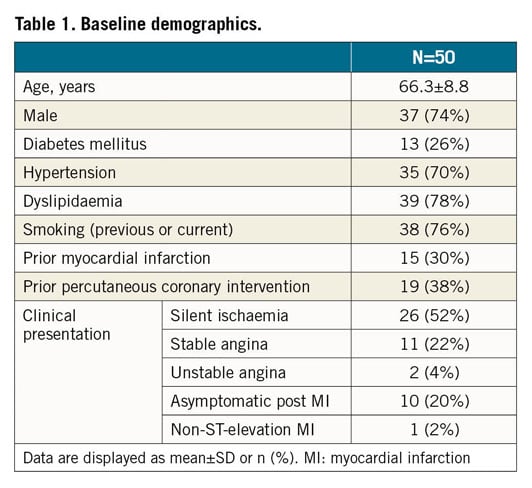
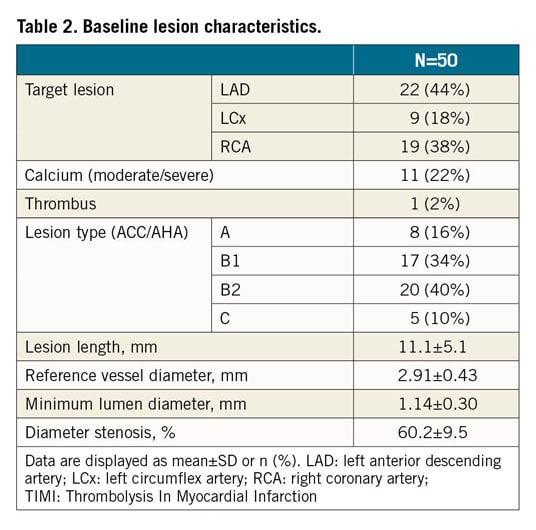
Fifty patients were enrolled (Figure 2). Per core laboratory assessment, reference vessel diameter was 2.91±0.43 mm and lesions were 11.1±5.1 mm long; 50% (n=25) were B2/C lesions (Table 1, Table 2).
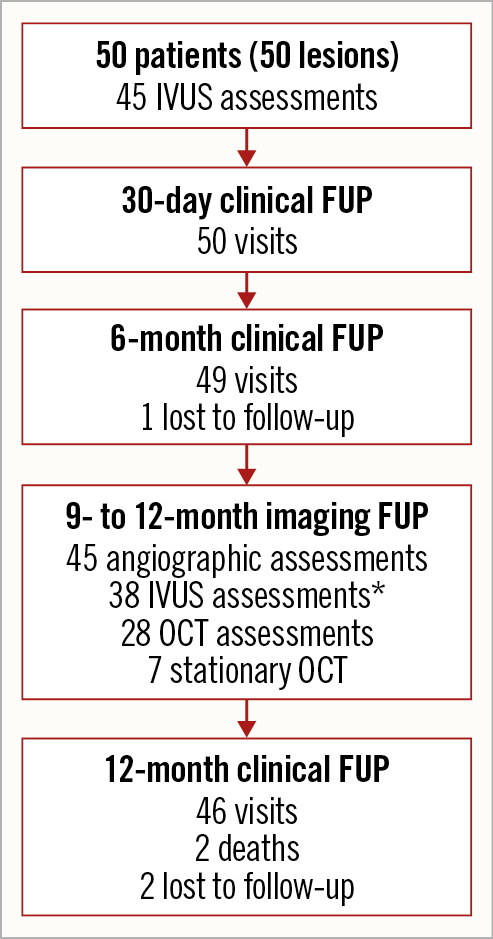
Figure 2. Patient flow chart. Two patients were lost to follow-up with last contact on day 81 and day 304. *Out of 45 IVUS assessments, seven could not be evaluated for paired analysis as post-procedure and follow-up time points were recorded with IVUS catheters recording at different ultrasound frequencies. IVUS: intravascular ultrasound; OCT: optical coherence tomography
Predilatation was performed in 96% of lesions (n=48) and post-dilatation in 62% (n=31). One patient (2%) required an additional bioadaptor to cover a vessel dissection. All devices could be implanted, and device success and procedure success were achieved in all patients. No periprocedural TV-MI was observed, but there was one non-TV-MI due to an occlusion of a side branch of a non-target vessel.
Up to 12 months, two TLFs occurred, both cardiac deaths. One patient with multiple medical comorbidities was found dead at home on day 255 post procedure. Another death on day 267 was caused by multi-organ failure following hospitalisation for heart failure (reported as not being due to ischaemic events) (Supplementary Table 5). There were no TV-MI, TLR, or definite or probable device thromboses (Table 3). Most patients (87.0%, 40/46) were on dual antiplatelet therapy at 12-month follow-up.
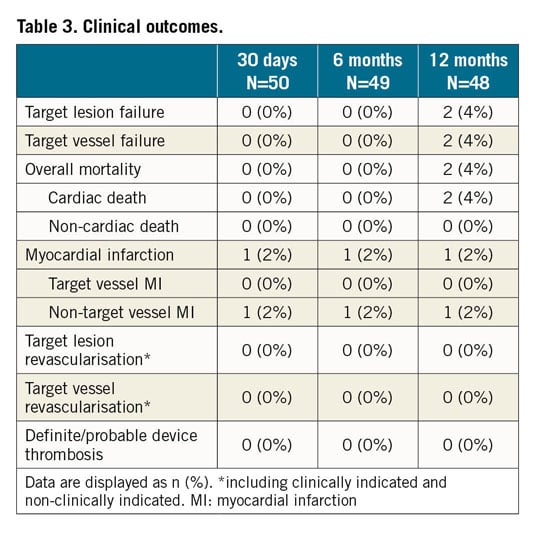
Per QCA core laboratory assessment, in-device acute gain was 1.61±0.34 mm and diameter stenosis was 5.4±8.4% post procedure. At a mean follow-up of 10.5±1.5 months, mean in-device late lumen loss was 0.12±0.18 mm and mean diameter stenosis 7.7±10.8% (Supplementary Table 6). Fourteen patients with post-procedural change in angulation of ≥9°9 showed a return towards baseline angulation at follow-up (from a mean of 157.5±14.5° post procedure to 149.7±16.1° at follow-up (Figure 3, Supplementary Figure 2).
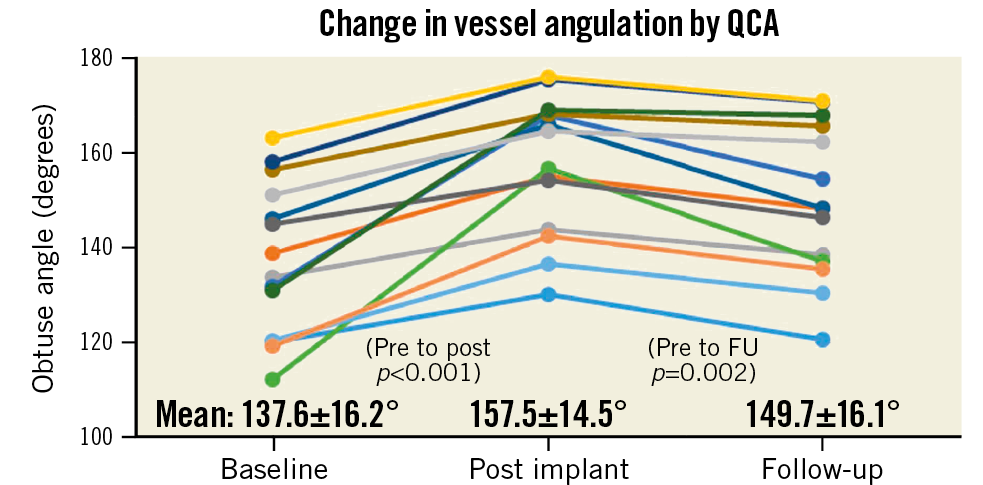
Figure 3. Change in vessel angulation by quantitative coronary angiography. Vessel angulation in 14 patients who had a ≥9° change of vessel angulation from baseline to post implant.
Mean IVUS follow-up was at 10.3±1.5 months. In paired analysis of 38 patients, from post procedure to follow-up, the mean bioadaptor area increased from 7.39±1.20 mm² to 7.74±1.46 mm² (Δ=5%, p=0.0005), the mean vessel area increased from 14.11±2.99 mm² to 14.54±3.12 mm² (Δ=3%, p=0.02), while the mean lumen area was maintained (7.39±1.20 mm² to 7.36±1.31 mm², Δ=0%, p=0.59) and the minimum lumen area decreased (6.10±1.15 mm to 5.86±1.20 mm, p=0.02) (Figure 4, Supplementary Table 7).
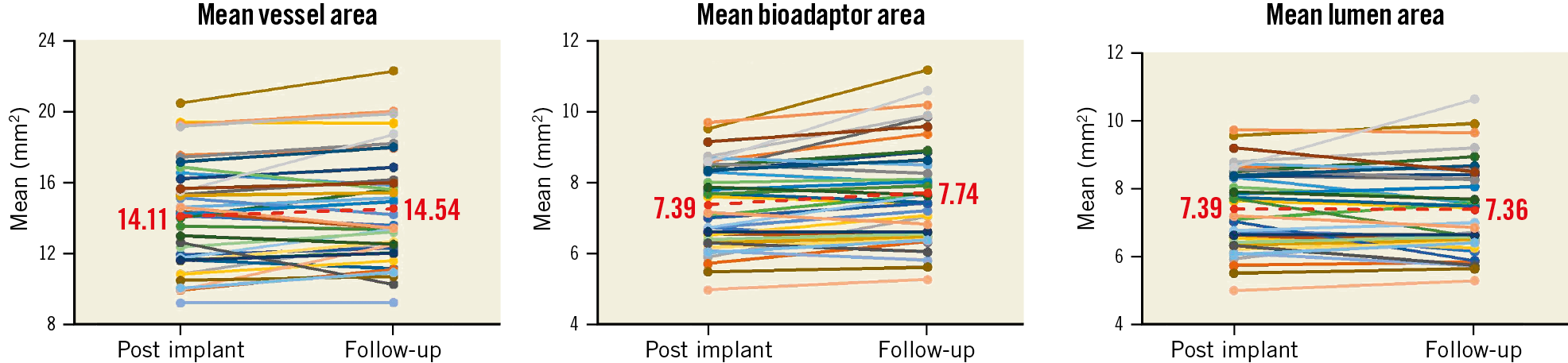
Figure 4. Paired intravascular ultrasound outcomes at follow-up compared to post procedure. Paired data are available for 38 patients.
OCT pullback was performed in a subset of 28 patients at a mean follow-up of 10.9±1.5 months. Qualitative analysis confirmed disengagement of the uncaging elements in all imaged segments (Supplementary Figure 1). Overall, the majority of struts (99.84±0.51%) were well apposed and fully covered by a thin, uniform neointimal layer of 140±40 µm (98.95±2.85% neointimal coverage). Complete OCT data at the cross-section and strut levels are provided in Supplementary Table 8. Stationary co-registered OCT performed in a subset of seven patients demonstrated pulsatility of the treated vessel segment during systole and diastole (Figure 5, Figure 6) with a lumen area change of 11% and a device area change of 7.3% (both are 95% predictive intervals relative to the mean).
Figure 5. Pulsatility analysis through stationary OCT recording. At follow-up, OCT recording was performed in seven patients by assessing the change during systole and diastole in the same co-registered stationary in-device spot. Each box plot represents the area changes per patient assessed during multiple cardiac cycles. The box plots themselves indicate median and interquartile ranges, and the ends of the whiskers represent maximum and minimum values excluding extreme results.
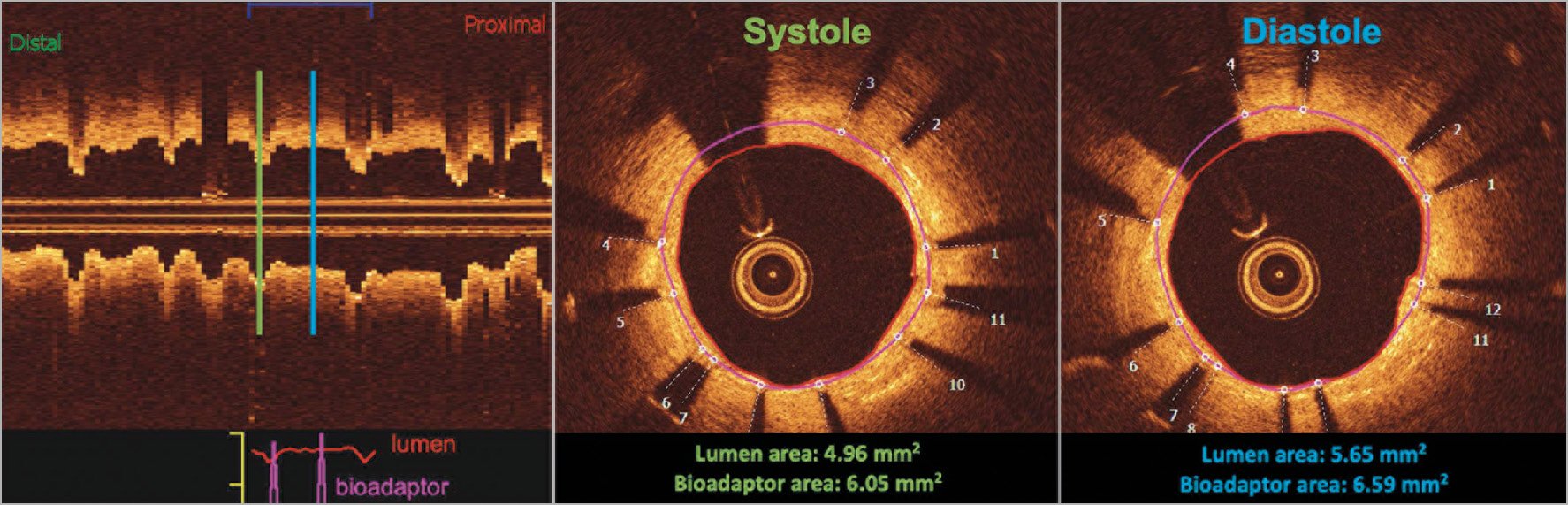
Figure 6. Case example of stationary OCT measurement during the cardiac cycle. The case example demonstrates the uncaging of the vessel, allowing vessel motion during the cardiac cycle. The longitudinal view on the left shows the consistent area changes between systole and diastole as measured at one stationary spot (lumen area Δ14% and bioadaptor area Δ8%). The purple line represents the bioadaptor area and the red the lumen area.
Discussion
The main findings of this study are that the novel bioadaptor demonstrated a) acute and 12-month safety and efficacy outcomes comparable to contemporary metallic DES, b) positive vascular remodelling10, cyclic pulsatility, and less geometric vessel distortion at follow-up, and c) safe disengagement of the uncaging elements.
The DynamX bioadaptor has been developed to overcome the limitations of rigid metallic stents that alter physiologic vascular dynamics by reducing vessel wall compliance, causing mechanical distortion of the vessel, and preventing positive adaptive remodelling. The bioadaptor was designed to have comparable characteristics with regard to thin struts, thin polymer coating, “limus” drug, visibility, deliverability, radial strength, and clinical performance to contemporary metallic DES. The unique design of uncaging segments provides the circumferential rings with freedom of radial and torsional motion allowing positive adaptive remodelling which can accommodate neointimal hyperplasia and/or neoatherosclerosis. The uncaging segments are initially bound by a bioresorbable polymer that resorbs over six months, a time point beyond which radial vessel support is not required, as previously demonstrated by bioresorbable scaffolds11.
CLINICAL EFFICACY AND SAFETY
Until the uncaging, the DynamX bioadaptor functions like a conventional stent with similar deliverability, conformability and radial strength. In particular, the acute gain (1.61±0.34 mm) and lumen diameter stenosis (5.4±8.4%) after implantation indicate a radial force comparable to other metallic stents12. Furthermore, the low in-device late lumen loss of 0.12±0.18 mm is comparable to latest-generation DES13.
Clinically, there were two TLFs, both cardiac deaths unrelated to the device or procedure. No other events were observed, resulting in a low TLF rate of 4%, comparable to contemporary conventional stents in similar populations12,13. The absence of any revascularisation might be a chance finding, or attributed to the low neointimal growth, the ability of positive remodelling to promote lumen area maintenance, and the cyclic pulsatility that allows the vessel to adapt to increased myocardial oxygen demand, e.g., during exercise10,14. Finally, the absence of definite or probable device thrombosis is favoured by the thin struts of the bioadaptor, which were well apposed and covered by a thin neointima layer.
MECHANISTIC INSIGHTS
The impact on vascular structure and function was assessed by IVUS, OCT and angiography.
Positive vascular or Glagovian remodelling of the vessel10 between implantation and follow-up was demonstrated by a vessel area increase of 3% and a device area increase of 5%. This increase enables the vessel to accommodate the neointima and maintain the lumen area unchanged, allowing the vessel to accommodate potential progression of disease. In comparison, the vessel area of contemporary stents ranged between a decrease of −2% and an increase of 1%, the device area ranged between 0% and 2%, and the lumen area decreased by −1% to −5% (Supplementary Table 9),15,16. Most importantly, the positive vascular remodelling did not come at the cost of strut malapposition.
Further, cyclic pulsatility was demonstrated by stationary co-registered OCT with a difference in cross-sectional lumen area of 11% between systole and diastole. To the best of our knowledge, no comparative OCT data are available in the literature. By IVUS, average changes in mean lumen area of 8-10% and isolated examples of up to 17% have been reported in predominantly disease-free coronary arteries, whereas segments with plaque or calcification showed smaller changes17,18,19. The observed lumen area change in DynamX-implanted vessels by OCT is thus within the range that has been reported for normal coronary arteries as measured by IVUS.
The 14 patients with ≥9° angulation change after a bioadaptor implantation, a threshold that has been associated with increased major adverse cardiac events and restenosis rates9, showed a return towards baseline angulation by ~50% at follow-up, demonstrating enhanced conformability and increased compliance of the uncaged bioadaptor device. In contrast, Gyöngyösi et al9 reported further straightening at follow-up compared to post procedure for conventional stents.
Indeed, the bioadaptor appears to provide the “promise” of what bioresorbable scaffolds once held without the hazards of previous bioresorbable scaffolds including limited radial strength, large profile and thick struts, cumbersome implantation technique, and bulk degradation with the potential for intraluminal scaffold dismantling over a prolonged period. Furthermore, pulsatility and positive remodelling appear to have been achieved after the uncaging process at six months, sooner than most bioresorbable scaffolds3,4,20.
UNCAGING
The safe disengagement of the uncaging elements was confirmed by a thin, uniform neointima layer with no evidence of struts protruding inside the vessel lumen by either IVUS or OCT, and absence of thrombotic events up to 12 months. Notably, finite element analysis of the bioadaptor has demonstrated reduced peak stress upon uncaging compared to a caged bioadaptor, suggesting a higher resistance to fracture after uncaging.
Limitations
Limitations include the small sample of patients with mainly stable angina and non-complex lesions. Data on less than 80% of patients were available for the primary imaging endpoint, and OCT was only performed at follow-up. Due to the absence of ECG gating, changes in the position of the OCT probe may have affected the pulsatility analysis. However, due to the high resolution of the OCT images, fiduciary landmarks to adjust for longitudinal movement of OCT catheters were used. Though perfect matching of the systolic and diastolic frames was not possible, any longitudinal mismatch that might have occurred is expected to be minimal and not of clinical relevance. Myocardial infarction was not determined according to current definitions, but as enzyme elevation of two times the upper normal limit of CK with elevation of CK-MB. Larger, randomised studies in more complex lesions are needed to compare the bioadaptor to contemporary DES and to verify whether the impact on vessel function results in a meaningful long-term clinical benefit.
Conclusions
Our study reports positive adaptive remodelling, cyclic pulsatility and return towards baseline vessel angulation after the implantation of the cobalt-chromium DynamX bioadaptor. The bioadaptor demonstrated acute performance and angiographic parameters similar to contemporary metallic DES with absence of TV-MI, TLR, and definite or probable device thrombosis to one year. Longer-term follow-up in comparative studies will show to what extent the bioadaptor may mitigate the annualised 2-4% device-oriented events that have been observed following the implantation of conventional metallic coronary stent prostheses.
|
Impact on daily practice This first clinical study provides data on the DynamX bioadaptor, a novel concept with uncaging elements that are released beyond six months after implantation, allowing a more physiological vascular response and thus potentially avoiding complications beyond one year attributed to caging of permanent stents. The present study confirmed the safety and feasibility of this novel concept with positive remodelling, cyclic pulsatility, low late lumen loss and low clinical event rates at 12-month follow-up. |
Acknowledgements
We thank Beatrix Doerr, medical writer, for her assistance in preparing this manuscript, reimbursed by Elixir Medical.
Funding
The study was funded by Elixir Medical.
Conflict of interest statement
S. Verheye reports personal fees from Neovasc and Biotronik outside the submitted work and speaker fees from Elixir Medical. B. De Bruyne reports that the Cardiovascular Center Aalst receives grant support from Abbott, Boston Scientific, and Biotronik AG and receives consulting fees on behalf of B. De Bruyne from Abbott and Boston Scientific outside the submitted work. B. De Bruyne is a shareholder in Philips, Siemens, GE, Bayer, HeartFlow, Edwards Lifesciences, and Celyad. D. Kereiakes reports other fees from Sino Medical Sciences Technology, Inc., Boston Scientific Corporation, Abbott Vascular, Svelte Medical Systems, Inc., Orchestra BioMed, Inc., Shockwave, and Elixir Medical Corporation, and personal and other fees from Ablative Solutions, Inc., during the conduct of the study. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

