Introduction
Percutaneous coronary intervention (PCI) in long coronary lesions still remains a challenge for the interventional cardiologist. Although the incidence of restenosis after drug-eluting stent (DES) implantation is relatively low, current literature indicates that stent overlapping is associated with major adverse cardiac events (MACE)1.
The novel BioMime™ (Meril Life Sciences, Vapi, Gujarat, India) 60 mm-long sirolimus-eluting coronary stent system with tapered design has recently been commercialised and is emerging as an interesting tool for patients with long and diffuse coronary lesions2,3. Therefore, the aim of the present work was to describe our initial experience with this new DES.
Methods
This is a prospective clinical cohort study which included 50 consecutive patients from February 2016 to April 2017 who underwent PCI, and in whom the implantation of a 60 mm-long DES was attempted due to the presence on angiography of coronary lesions longer than 48 mm. During the enrolment period, up to 21 potentially eligible patients were not included due to the operators’ decision. Written informed consent was obtained from all patients. PCI was performed according to standard hospital practice and current guidelines. Interventional and pharmacological strategies were left to the discretion of the operator. The optimal PCI result was defined as a post-PCI diameter stenosis <20% by visual estimation. We expressed continuous variables as mean±standard deviation or median (interquartile range [IQR]), and discrete variables as percentages. MACE during follow-up (defined as a composite of death, myocardial infarction and/or target lesion revascularisation) were adjudicated by independent researchers blinded to patient characteristics.
Results
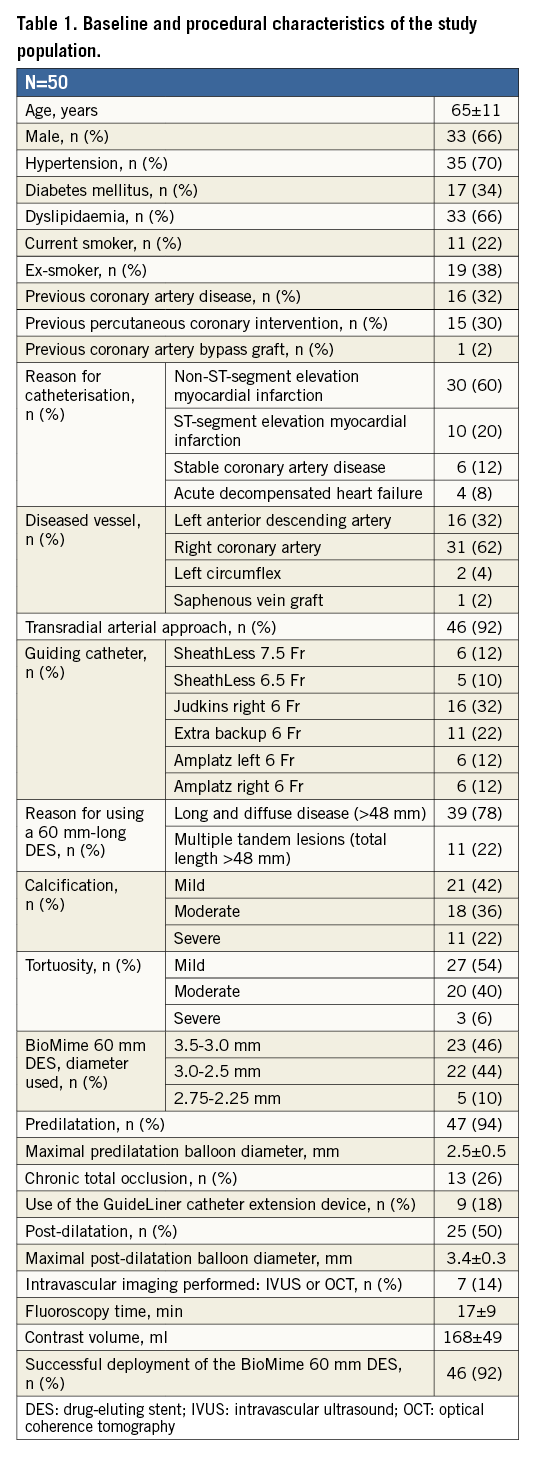
The baseline characteristics of the study population and procedural data are described in Table 1. Mean patient age was 65±11 years, and 66% were male. Non-ST-segment elevation myocardial infarction was the main indication for catheterisation (60%) and the right coronary artery was the most frequently treated vessel (31/50, 62%). The transradial approach was used in 46 (92%) cases, choosing a SheathLess Eaucath® guiding catheter (Asahi Intecc, Aichi, Japan) in 11 of them (22%). Thirty-nine patients (78%) exhibited long coronary lesions (>48 mm) on angiography, while the remaining 11 patients (22%) presented multiple severe tandem lesions involving arterial segments longer than 48 mm. All were type C lesions, as many as 29 cases (58%) showed moderate-severe calcification, and 13 cases (26%) presented chronic total occlusions.
The BioMime 60 mm DES was successfully deployed, with an optimal angiographic result in 46 (92%) patients (Figure 1). A guide extension catheter (GuideLiner®; Vascular Solutions, Inc., Minneapolis, MN, USA) was used in nine of them (18%). In four patients, the 60 mm stent deployment failed, because of the impossibility of crossing the lesions completely with the devices, and the need to overlap shorter DES to achieve an optimal final result. The mean total fluoroscopy time was 17±9 minutes and the mean total amount of contrast per procedure was 168±49 ml. During a median follow-up of 275 days (IQR 166-386), no MACE were observed; two patients (4%) were readmitted complaining of chest pain with normal troponin levels. In these patients, new catheterisations were performed revealing the optimal angiographic result of the previously deployed BioMime DES and ruling out the need for new revascularisations.
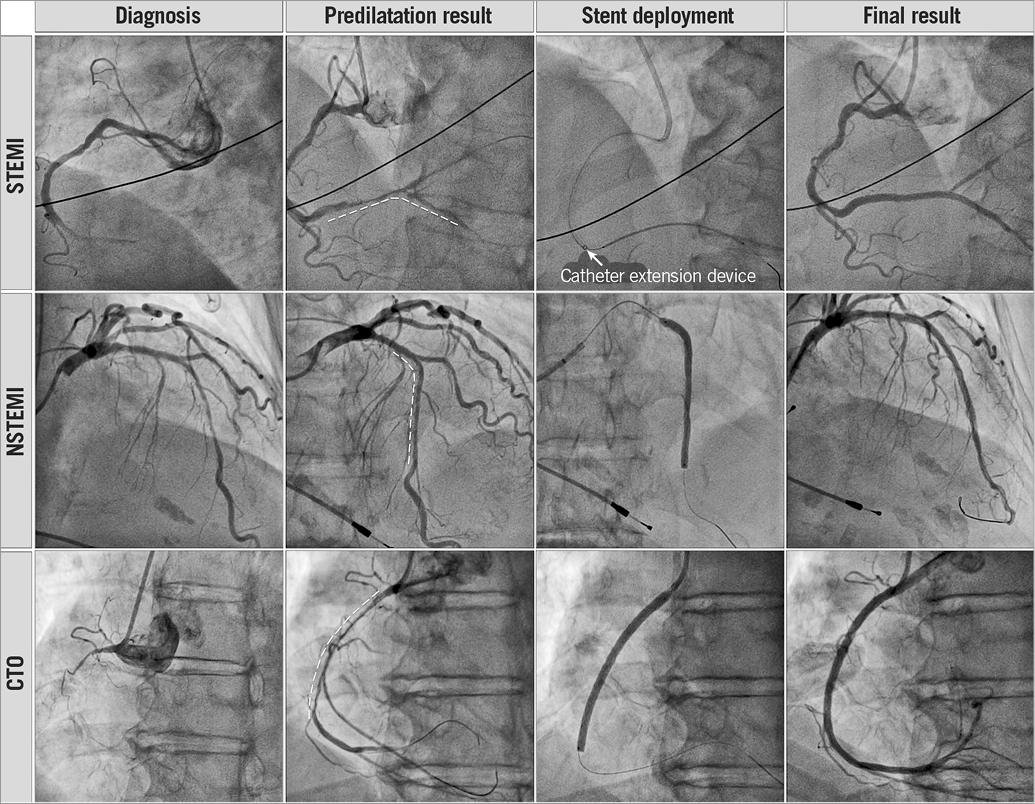
Figure 1. Angiograms of patients in three different clinical scenarios in whom the BioMime 60 mm-long tapered DES was successfully deployed.
Discussion
Treatment of long coronary lesions with stent overlapping is common and has been shown to be time-consuming and associated with a significant increase in material expenditure4. Indeed, when coronary lesions involved segments >48 mm, the only possibility for the interventionalist was to overlap two or more stents. Overlapped portions tend to have a greater neointimal proliferation, make the vessel rigid due to the excess of metal, are more prone to stent fracture, and cause higher vascular injury and delayed healing, circumstances that lead to more restenosis. Moreover, an increase in side branch jailing due to the presence of a double physical layer of stent struts increases the probability of new revascularisations during follow-up1,4. In addition, multiple stenting procedures increase operator exposure to radiation, and increase the amount of contrast media administered to the patient. Therefore, the use of a single long DES could, at least theoretically, improve these issues. In this regard, BioMime is the world’s first commercialised tapered 60 mm DES system with an indication for the treatment of very long lesions and, to the best of our knowledge, this is the first report describing the initial experience with this novel DES.
In our case series, we managed to deploy the BioMime successfully in 92% of the cases where it was attempted. We believe these are promising results given the unfavourable anatomic context. However, in 18% of the cases, the use of a guide catheter extension device was required to advance the DES across the lesion. In four patients, we failed to implant the device despite thorough predilatation and the use of extra-support techniques. All of these patients exhibited a moderate-severe calcification disease together with a moderate-severe tortuosity. In order to overcome these difficulties and achieve an optimal angiographic result, finally we overlapped several shorter DES in these patients. It is important to highlight that we observed no angiographic distal vessel dissection, probably due to the tapered design of the device (distal diameter is smaller than proximal), which avoids stent size mismatch with the distal coronary lumen. Moreover, all the procedures were performed with an acceptable mean fluoroscopy time and mean contrast volume per procedure when compared to other series reported in the treatment of long coronary lesions.
Limitations
Some limitations need to be addressed. Firstly, this was a small observational study that included a limited number of patients and only aimed to describe our initial experience with this novel DES. Randomised studies with larger series and better controlled scenarios are needed to confirm our results regarding the efficacy and safety of this new stent device for the treatment of long coronary lesions. Secondly, since patients were probably highly selected and due to the absence of clearly defined inclusion/exclusion criteria, there was probably a selection bias. Finally, significant interobserver variability could exist since quantitative coronary angiography analysis was not carried out.
Conclusions
According to our initial experience, the use of the novel BioMime 60 mm-long DES for the treatment of very long coronary lesions exhibits promising results in a relatively short to intermediate follow-up. If our results are reproduced in larger and longer studies, we envisage its use as a potentially useful tool.
| Impact on daily practice The present study confirms that the novel BioMime 60 mm-long sirolimus-eluting tapered stent system may be an appropriate alternative to multiple stent implantation for long coronary lesions. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

