
Abstract
Aims: The aim of this study was to evaluate the functional result immediately post PCI and at nine-month follow-up, and to ascertain how often a functionally optimal result of >0.95 can be achieved in long coronary lesions treated with long second- or newer-generation DES.
Methods and results: Patients receiving DES measuring ≥30 mm with FFR value ≤0.8 were included in the study. Stent length was defined as long (30 to 49 mm; L-DES) and ultra-long (≥50 mm; UL-DES). Angiographic and FFR evaluation was performed before and after PCI and at nine-month follow-up. A total of 74 patients each received a mean stent length of 50.72±14.6 mm. Mean FFR post PCI was 0.88±0.06. An optimal post PCI FFR value of >0.95 was achieved in only 9/74 patients (12.2%), and was not achieved in any UL-DES patients. Only 12/74 (16.2%) had FFR post PCI of 0.91 to 0.95; 8/74 (10.8%) patients remained ischaemic (≤0.8). FFR gradient across the stent was higher in UL-DES patients compared to L-DES patients (0.07±0.03 vs. 0.04±0.03; p=0.001). At follow-up, the angiographic restenosis rate was 4.7%, and the functional restenosis rate was 15.1%.
Conclusions: The FFR result post PCI was suboptimal in the majority of patients treated with long DES and was particularly poor when the total stent length exceeded 50 mm.
Abbreviations
BMS: bare metal stents
CABG: coronary artery bypass graft surgery
DES: drug-eluting stent
FFR: fractional flow reserve
FU: follow-up
IVUS: intravascular ultrasound
MACE: major adverse cardiovascular events
PCI: percutaneous coronary intervention
QCA: quantitative coronary angiography
SD: standard deviation
STEMI: ST-elevation myocardial infarction
TLR: target lesion revascularisation
Introduction
Fractional flow reserve (FFR)-guided percutaneous coronary intervention (PCI) has been shown to improve clinical outcomes in patients when compared to angiographically guided PCI at long-term follow-up1,2. There is an increasing body of evidence that FFR measured post PCI is a strong independent predictor of major adverse cardiovascular events (MACE). Pijls at al have shown that the higher the post-PCI FFR following bare metal stent (BMS) implantation, the lower the MACE rate at six-month follow-up3. Specifically, it seems that the optimal PCI result is achieved when the FFR value post PCI is >0.95, comparable to that of angiographically normal coronary arteries4. In support of this, a recent meta-analysis by Johnson et al again showed that the higher the FFR post PCI, the better the prognosis5.
An important aspect of the previous evidence is that the stent length was relatively short, averaging less than 20 mm. However, the average stent length in current practice appears to be longer, due to the improved performance of DES. Despite consistent evidence that second-generation stents significantly reduce TLR compared to BMS or first-generation DES6,7, MACE rates remain remarkably high when long stents are used8,9. It remains unclear whether the functional result achieved post PCI is equally acceptable when using long (>30 mm) or ultra-long (>50 mm) stents.
The assessment of restenosis has traditionally been done using angiographic follow-up or, increasingly, clinically driven restenosis. This may not be ideal when we consider that the angiographic result post PCI may not be functionally acceptable. One might speculate that the traditional evaluation of restenosis may well underestimate the actual rates of TLR, especially in long lesions treated with long DES. Currently, there is no study that has used FFR to assess restenosis at long-term follow-up in patients with long diffuse disease.
Therefore, the aim of this study was to evaluate the functional result immediately post PCI and at nine-month follow-up, and to ascertain how often a functionally optimal result of >0.95 can be achieved in long coronary lesions treated with long second- or newer-generation DES.
Methods
This was a prospective, “all comers” single-centre pilot study. The study protocol was approved by the local ethics committee and informed consent was obtained in all cases.
Consecutive patients admitted with stable angina pectoris or an acute coronary syndrome, with a lesion in a major epicardial vessel, were enrolled. Patients were included if the FFR value was ≤0.8 and it was envisaged that a stent with a length ≥30 mm would be necessary. Patients with ST-elevation myocardial infarction (STEMI), or in whom long-term dual antiplatelet therapy was contraindicated, or with an expected survival <1 year, or with an allergy to biolimus, everolimus or zotarolimus were excluded.
DEFINITION OF STENT LENGTH
Based on previous work, stent length was defined as long (30 to 49 mm; L-DES) or ultra-long (≥50 mm; UL-DES)9. Quantitative coronary angiography (QCA) was retrospectively measured in all cases according to a standard protocol using QAngio® XA 7.3 software (Medis medical imaging systems bv, Leiden, The Netherlands).
FFR PROTOCOL
A functionally significant result was defined as an FFR value ≤0.8 as per previous work using a PressureWire™ Certus™ (St. Jude Medical, St. Paul, MN, USA)2. FFR was measured according to standard practice, with maximal hyperaemia induced using an intravenous infusion of adenosine at a rate of 140 μg/kg/min after administration of 200 mcg of intracoronary nitroglycerine. The following measurements were performed before and after PCI (Figure 1):
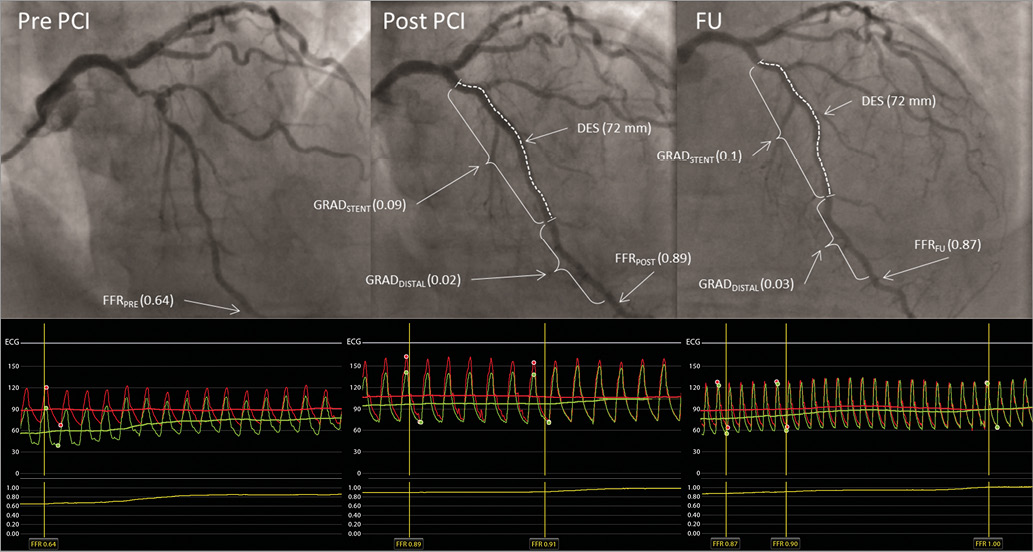
Figure 1. FFR measurement (example).
1. Baseline (FFRPRE) - defined as evaluation of lesion significance prior to PCI, with the pressure wire sensor positioned at the beginning of the distal segment of the artery.
2. Post PCI:
– FFRPOST - post PCI the FFR was measured in the same position as FFRPRE.
– FFR gradient:
- FFR gradient across the stent (GRADSTENT) was defined as the difference between the FFR value just proximal to the stent and the FFR value just distal to the stent.
- FFR gradient distal to the stent (GRADDISTAL) was defined as the difference between the FFR value just distal to the stent and the FFR value at the beginning of the distal segment.
- Total gradient was defined as the difference between the FFR value just proximal to the stent and the FFR value at the beginning of the distal segment.
3. The same FFR values were obtained at nine-month follow-up (FFRFU, GRADStentFU, GRADDistalFU).
All study lesions were treated with Biolimus A9™ (BioMatrix Flex™; Biosensors, Newport Beach, CA, USA), everolimus (XIENCE Xpedition®; Abbott Vascular, Santa Clara, CA, USA) or zotarolimus (Resolute Integrity®; Medtronic Vascular, Santa Rosa, CA, USA) drug-eluting stents.
Angiographic success was defined as TIMI 3 flow with a residual angiographic diameter stenosis of ≤10%. The aim was to achieve the optimal result, defined as an FFRPOST ≥0.95. The operators were encouraged to post-dilate in every case; however, where there was an FFRPOST <0.95, further post-dilatation was mandatory. If there was clear evidence of atheroma beyond the stented segment, then the operator was encouraged to try to optimise the functional result further by implanting another stent more distally.
All patients had medical therapy optimised (as tolerated), with all receiving dual antiplatelet therapy for a period of 12 months. Patients were planned to undergo angiographic and FFR follow-up at nine months.
STUDY DEFINITIONS
An optimal functional result was defined as an FFR value post PCI of >0.95, and a desirable result as an FFR value of >0.90 to 0.95. An FFR value ≤0.80 found at nine-month follow-up was defined as functional restenosis. Patients in whom the FFRPOST was ≤0.80 immediately post PCI were excluded from the follow-up analysis of functional restenosis (Figure 2).
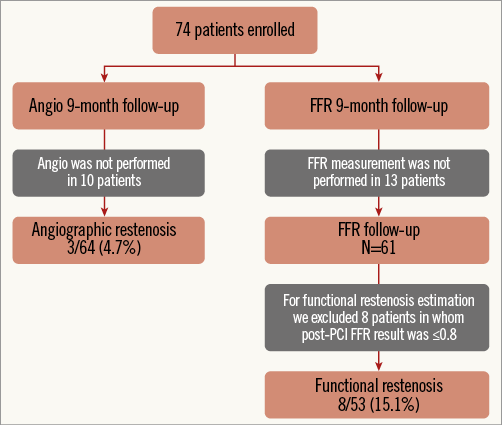
Figure 2. Patient flow chart.
Angiographic restenosis was defined as a >50% diameter stenosis at follow-up, as measured by QCA. Target lesion revascularisation (TLR) was defined as any repeated percutaneous intervention of the target lesion or bypass surgery of the target vessel performed for restenosis or other complication of the target lesion10.
STATISTICAL ANALYSIS
Analysis was performed according to the intention-to-treat principle. Normal distribution was tested using the Kolmogorov-Smirnov test. Continuous variables with a normal distribution were compared using a Student’s t-test and presented as mean±standard deviation (SD); otherwise, non-parametric Wilcoxon signed-rank tests were used. Categorical variables were compared by using a χ2 or Fisher’s exact test and are presented as numbers or percentages. Multiple groups were compared by using a non-parametric Kruskal-Wallis test. A p-value <0.05 was considered significant. Statistical analysis was performed with SPSS, Version 20.0 (IBM Corp., Armonk, NY, USA).
Results
Between October 2011 and November 2013, a total of 74 patients were enrolled in the study. Baseline demographic, clinical, lesion, and procedural characteristics are listed in Table 1, Table 2, and Table 3, respectively. Angiographic and functional assessment by FFR was performed in all 74 patients immediately pre and post stent implantation. At nine months, coronary angiography was repeated in 64/74 (86.5%) patients with FFR measurement in 61/74 (82.4%) (Figure 2). The mean stent length was 50.7±14.6 mm (ranging between 30 mm and 98 mm), with a median stent length of 49 mm, indicating that this was indeed a cohort with long diffuse coronary artery disease.
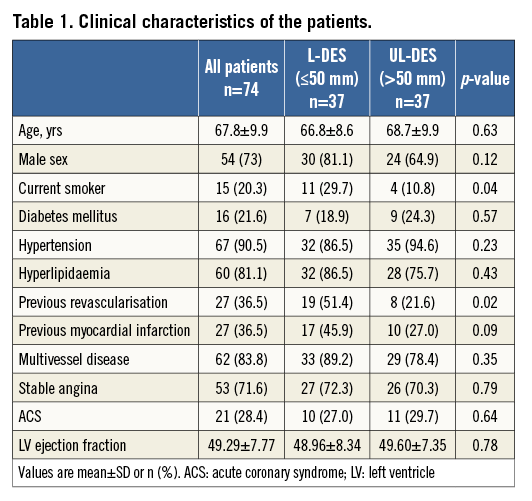
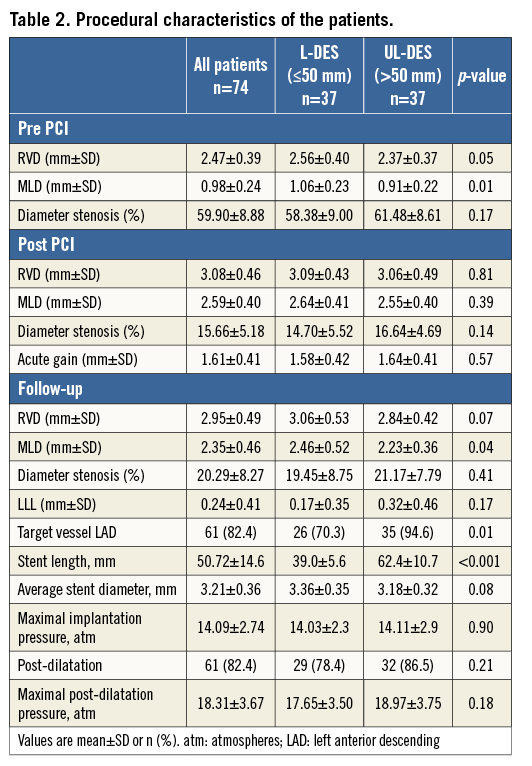
BASELINE AND POST-PCI FUNCTIONAL ASSESSMENT
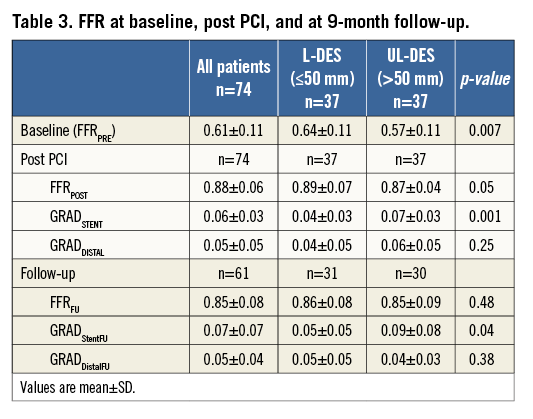
At baseline, the mean FFRPRE was 0.61±0.11. The mean FFRPOST immediately after the PCI increased to 0.88±0.06 (p<0.001). An optimal FFR value of >0.95 was achieved in only 9/74 patients (12.2%). Only 12/74 (16.2%) had a desirable FFRPOST of 0.91 to 0.95 (inclusive). Finally, 53/74 (71.6%) had FFR values ≤0.90. In 8/74 (10.8%) of the patients, the FFR remained haemodynamically significant at ≤0.8 (mean FFRPOST 0.77±0.04, mean stent length 50±15 mm), indicating significant inducible ischaemia (Figure 3, Table 3).
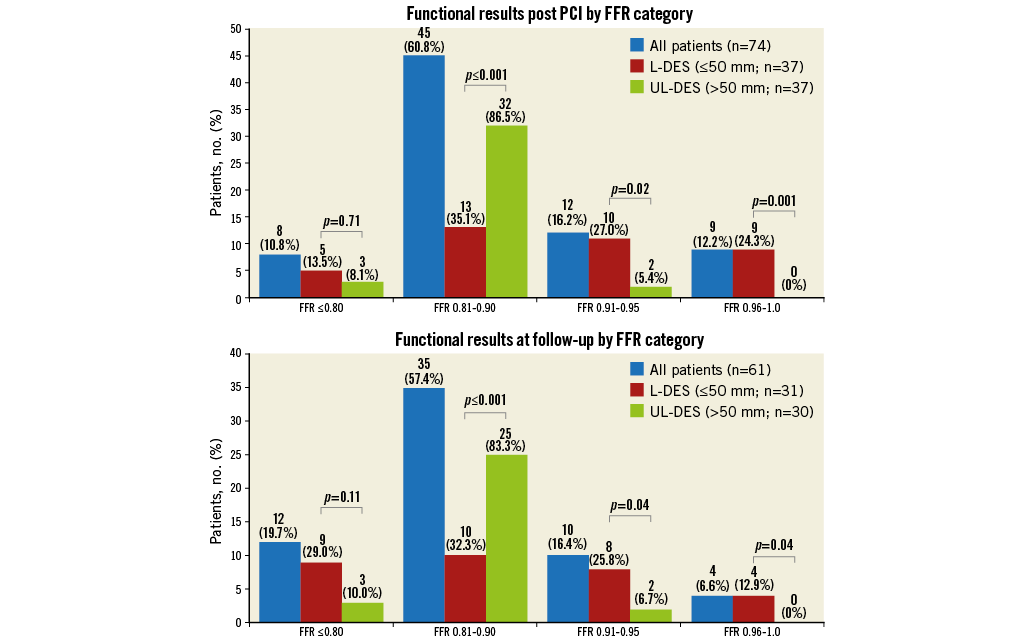
Figure 3. Functional results post PCI and at follow-up by FFR category.
FFRPOST IN RELATION TO STENT LENGTH
At baseline, the mean FFRPRE value was significantly lower in the UL-DES group compared to the L-DES group (0.57±0.11 vs. 0.64±0.11, p=0.007). Post PCI, the FFRPOST value remained numerically lower in the UL-DES group compared to the L-DES (0.89±0.07 vs. 0.87±0.04, p=0.05). An optimal FFR value of >0.95 was not achieved in any of the UL-DES patients (Table 3). A desirable FFRPOST value of >0.90 was achieved in only 21/74 patients (28.4%), of whom 19 were in the L-DES group and only two in the UL-DES group, suggesting that even a desirable result is difficult to achieve in patients receiving ultra-long stents. There was a low and inverse correlation between stent length and FFR post PCI (r=-0.297, p=0.018).
FFR GRADIENT
The position of the pressure wire sensor is crucial; therefore, the pullback gradient was measured. In the entire cohort, the mean FFR GRADStent was 0.06±0.03 and the FFR GRADDistal was 0.05±0.05. The total gradient in patients who achieved an optimal result of FFRPOST of >0.95 post PCI was 0.02±0.02. Patients who remained haemodynamically significant post PCI (i.e., FFR ≤0.8) had a total gradient of 0.19±0.09. In these patients, the GRADDistal was significantly higher than the GRADStent (0.11±0.07 vs. 0.08±0.02, p<0.0001) (Table 3), possibly indicating more diffuse disease. The GRADStent was significantly higher in the UL-DES group compared to the L-DES group (0.07±0.03 vs. 0.04±0.03, p=0.001).
AT NINE-MONTH FOLLOW-UP
A total of 61/74 patients completed functional follow-up at nine months. The FFR values of these 61 patients were compared post PCI and at nine months. A desirable FFR value of >0.90 was achieved in 16/61 patients (26.2%) post PCI, which decreased to 14/61 patients (23%) at nine-month follow-up. A total of 8/61 patients (13.1%) continued to have haemodynamically significant disease, which increased to 12/61 patients (19.7%) at nine-month follow-up. The restenosis rate was 4.7% (3/64) by angio and 15.1% (8/53) by functional assessment (Figure 4). The TLR rate was 8.1% (6/74). One patient died during the follow-up period.
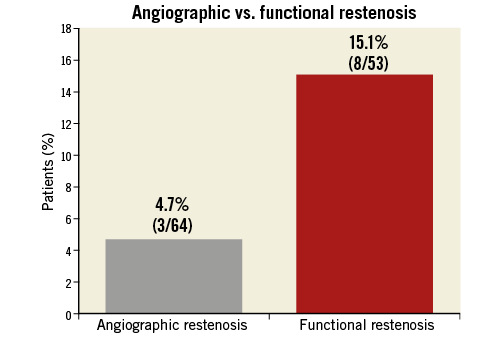
Figure 4. Angiographic vs. functional restenosis.
Discussion
To our knowledge, this is the first study that has measured FFR post PCI exclusively in patients treated with long (>30 mm) and ultra-long (>50 mm) newer-generation DES and with long coronary lesions. There are several key findings of this study, the first of which is that achieving an optimal post-PCI FFR result of >0.95 is only possible in a limited number of cases, equating to 12.2%. In particular, an optimal FFR result of >0.95 could not be achieved in any patient who was treated with a stent >50 mm in length. Furthermore, the use of long and ultra-long stents resulted in a high proportion of patents with residual haemodynamically significant ischaemia, defined as an FFR ≤0.80, despite optimisation of the stent during the procedure. Finally, the rate of functional restenosis at nine-month follow-up was shown to be approximately three times higher than the rate of angiographic restenosis in this cohort.
This study evaluated the functional results that can be achieved in patients treated with at least second-generation drug-eluting stents for diffuse long coronary artery lesions. These data show that achieving a functionally optimal or even desirable result in long coronary artery disease using long coronary stents can be very difficult, even when using newer-generation drug-eluting stents. Previous work shows that the measurement of FFR post PCI is an important predictor of MACE. Pijls et al have shown that the higher the FFR value post PCI, the lower the rate of MACE3. This has been subsequently corroborated from a meta-analysis performed by Johnson et al, suggesting that FFR provides important prognostic information5.
There are several key differences between our findings and those seen in previous studies. Crucially, the meta-analysis by Johnson et al showed that only 27% of the patients treated received a drug-eluting stent as opposed to all patients in our cohort. Obviously, the former is not reflective of current practice, as second-generation drug-eluting stents and beyond are now more widely used, largely driven by the fact that angiographic restenosis is less of an issue compared to the levels of angiographic restenosis seen during the era of BMS and even first-generation DES6. In the first studies to look at the predictive value of FFR, the stent length ranged on average between 16 and 20 mm, and desirable results were achieved in a very significant proportion of patients who had PCI3,11,12.
There are several lines of evidence to suggest that the optimal PCI result is achieved when the FFR value post PCI is >0.95, comparable to that found in angiographically normal coronary arteries (0.97±0.02 [0.92-1.0])13. Moreover, the evidence also shows that optimal FFR values >0.95 are associated with lower rates of MACE3,5,11,12. In patients receiving bare metal stents, Pijls et al showed that the six-month MACE rate was 4.9% when the FFR value was >0.95, compared to 5.5% when the FFR value was between 0.91 and 0.95. MACE rates increased significantly to 18.1% when the FFR value was between 0.81 and 0.9 (a threefold increase), and as high as 30% when the FFR value remained ≤0.803. Subsequent studies have reproduced Pijls’ findings but with a higher proportion of patients achieving FFR values >0.9511,12. An important difference in these more recent studies has been the higher use of drug-eluting stents. With these cut-offs in mind, we see that an optimal result, defined as an FFR value of >0.95, was achieved in only 36% of the patients in the Pijls et al trial, in contrast to only 12% in the current study. Even when we redefine a desirable post-PCI FFR threshold as >0.90, then the proportion rises to 68% in the Pijls study; however, it remains disappointingly low at 28% in the current study despite aggressive post-dilatation. This notable difference may be explained in part by the considerable additional diffuse atherosclerotic burden as well as the stent length. The average stent length is approximately threefold longer in our study compared to that seen in the Pijls study and that of the subsequent studies mentioned above. This suggests that a functionally suboptimal result as measured by FFR is much more likely to occur if one decides to treat long diffuse coronary artery disease with long drug-eluting stents, even when these are newer-generation DES. In every case where patients received a stent of >50 mm in length, an optimal FFR result >0.95 could not be achieved in this study. Similarly, there were only two cases where patients achieved a desirable FFR value of >0.90 but ≤0.95, indicating that the goal of achieving a functionally optimal or even desirable result post PCI in long coronary artery disease was virtually impossible. In support of this, Honda et al suggested that ultra-long (>50 mm) second DES implantation should be avoided due to a higher risk of TLR (13.5%) at long-term follow-up (23.2±13.2 months) relative to long (20-50 mm) second DES (TLR 7.2%) and short (<20 mm) DES (TLR 6.0%).
In contrast to achieving an optimal or desirable result post PCI, there remains a haemodynamically significant cohort (FFR ≤0.80) in all study populations. The proportion of these patients was approximately 6% in the Pijls study, rising to 11% in patients with long diffuse coronary disease, as was the case in the current study. Pijls postulated that the persistent ischaemic burden seen in his cohort probably reflected the underappreciated nature of diffuse disease that remains in the unstented segment of the coronary artery. This again is the most likely explanation in our population and, in support of these findings, we found that the gradient in the distal vessel (i.e., the residual gradient distal to the stent) increased significantly as FFR values post PCI decreased. Furthermore, and in contrast to previous studies14, the gradient across the stented segment itself actually increased as FFR values decreased, despite aggressive post-dilatation. Notably, in the group of patients who remained ischaemic (i.e., those with an FFR ≤0.80), the gradient along the entire vessel was significantly higher than in the group of patients who had FFR values >0.80. In the group with residual ischaemia, the gradient in the distal vessel itself was the greatest contributor to the total gradient being significantly higher than the gradient across the stent, probably again reflecting the diffuse atherosclerotic burden in the entire vessel. In these patients, it may be that, despite second-generation DES use, functionally optimal or even desirable results cannot be achieved; however, it may be that adjuvant use of IVUS or OCT imaging could provide insight into the degree of atherosclerotic burden as well as the causes of this residual in-stent FFR gradient and guide us as to how to improve functional results. In support of this, focal underexpansion of stents can be seen in as many as 35% of PCI cases when a stent >28 mm is used15. Further support for this strategy comes from Fearon et al, who showed that the absence of a hyperaemic residual pressure gradient is a prerequisite for optimal stent deployment. In this study, the FFR gradient was higher in the UL-DES group (0.07±0.03) in comparison to shorter stents (0.04±0.03). This may reflect the altered shear environment within the long stented segment, thereby possibly leading to MACE; however, a much larger study will be needed to prove this theory.
Historically, many studies used angiographic assessment for follow-up. In this study, following angiographic assessment at nine months, we found that there was a restenosis rate of 4.7%, in keeping with previous studies that have used second-generation DES16. However, in this study, we also performed a haemodynamic assessment at nine months and found a functional restenotic rate of 15.1%, equating to a threefold to fourfold increase. This suggests that angiographic assessment potentially misses a significant proportion of patients, and therefore we should not be surprised if MACE rates are higher, particularly in cases where PCI has been performed in long diffuse coronary disease using long second-generation DES. Simply believing the angiographic result may not be sufficient in order to avoid MACE; functional restenosis may be a better predictor of MACE.
While there have been studies using IVUS guidance to optimise stent implantation, thereby increasing minimal luminal diameter, this gain has not been shown to achieve functionally better results, as FFR was not measured. In the AVIO study, the increase in MLD did not improve the clinical endpoint17. Honda et al reported a 13.5% TLR rate in a patient group with stent length >50 mm (UL-DES) vs. 6.0% in a short stent group (<20 mm) vs. 7.2% in 20-50 mm (L-DES), despite IVUS guidance9. In a recent study, Elgendy et al reported that IVUS-guided PCI is superior to angiography-guided PCI in reducing the risk of major adverse cardiac events, primarily because of reduction in the risk of ischaemia-driven target lesion revascularisation18.
Some of the findings of this work may be explained by the fact that the majority of cases were diffuse disease in the LAD. One might speculate that, had there been a higher proportion of patients with RCA or LCx disease, more optimal results would have been achieved19.
Limitations
Our study shows a very significant rate of functional restenosis; however, we cannot say what impact this would have on MACE. Nevertheless, when we examined previous studies, we found that the rate of MACE was consistently higher than the rate of angiographic restenosis. One could speculate that this difference is driven by the residual ischaemic burden that has up to now been underappreciated. Even though IVUS-guided PCI has not to date been shown to improve functional results, the lack of IVUS and OCT utilisation in the current study is a major limitation and probably warrants re-evaluation, particularly in the context of patients with long diffuse coronary lesions treated with long and ultra-long stents.
Conclusion
The FFR result post PCI is suboptimal in patients with long diffuse coronary artery disease treated with long second- or newer-generation DES, and is particularly poor when the total stent length exceeds 50 mm. The current study shows that in the majority of cases diffuse coronary artery disease cannot be effectively treated using long and ultra-long drug-eluting stents.
| Impact on daily practice The current study shows that in the majority of cases diffuse coronary artery disease cannot be effectively treated using long drug-eluting stents. In patients with diffuse coronary artery disease the option of CABG should be considered, particularly when the total lesion length exceeds 50 mm. |
Conflict of interest statement
The authors have no conflicts of interest to declare.

