
Heart valve replacement remains one of the commonest operations performed all over the world and can be regarded as a medical and engineering marvel. However, the search for the perfect solution continues as none of the currently available prostheses satisfies the “10 commandments” set by Dwight Harken in 19621. These are as follows:
1. It must not propagate emboli.
2. It must be chemically inert and not damage blood elements.
3. It must not offer resistance to physiological flow.
4. It must close promptly (less than 0.05 s).
5. It must remain closed during the appropriate phase of the cardiac cycle.
6. It must be inserted in a physiologic site, generally the normal anatomic site.
7. It must be capable of permanent fixation.
8. It must not annoy the patient.
9. It must be technically practical to insert.
10. It must have lasting physical and geometric features, i.e., last lifelong.
None of the currently available valves provides a one-time solution for the patient.
Issues associated with current surgical prostheses
A mechanical prosthesis in combination with adequate anticoagulation can last lifelong and satisfy the majority of these criteria. However, problems associated with noise, damage to blood elements and issues associated with anticoagulation pose challenges and hence the popularity of its use is diminishing. Further, redo surgery is the only option to treat any failure associated with the mechanical valve2.
The other alternative is a bioprosthetic valve, i.e., tissue valve. The main reason for the popularity of the bioprosthetic valve is its freedom from anticoagulation; however, whilst it wins on this front it loses on durability. Despite significant improvements from the first to the third generation of tissue valves, durability remains far from satisfactory, especially in the mitral position and in younger patients. More than 250,000 bioprosthetic valves are implanted annually and the number is on the rise3. With such large numbers of bioprosthetic valves being implanted, the lowering of the cut-off age for bioprosthetic implantation, and an ageing population, one can expect an increase in the number of valves experiencing structural valve deterioration (SVD). Redo operations carry an increased risk of operative mortality. The operative risk of elective redo open valve surgery has been reported to range from 2%-7%, but can be greater than 30% in high-risk, non-elective patients3. Thus, bioprosthetic valves also do not provide a one-time solution2.
Valve-in-valve (VIV)
The success of transcatheter aortic valve implantations (TAVI) has introduced another class of bioprosthetic valves. The essential feature of these valves is the ability to implant them under imaging guidance without the need for open surgery. Transcatheter heart valves (THV) have also been successfully used to treat inoperable or high-risk patients with failing bioprosthetic valves, referred to as VIV4. This treatment is an attractive option as it avoids redo sternotomy and cardiopulmonary bypass and can potentially accelerate patient recovery and reduce length of hospital stay. VIV is now increasingly performed to treat bioprosthetic valve failure in the aortic, mitral, tricuspid and pulmonic positions4.
In theory, it is conceivable that a younger patient will have a bioprosthesis rather than a mechanical valve and, when presented with failure, will proceed to a percutaneous VIV implantation. This would, in theory, allow a patient an anticoagulation-free, near perfect solution for three decades.
Issues associated with VIV
Malposition of the THV can result in embolisation, suboptimal function or a leak. In the aortic position, coronary obstruction and residual high gradient are the two main issues5. In the mitral position, left ventricular outflow tract obstruction (LVOTO) and delayed embolisation are the main concerns6,7. The balloon-expandable SAPIEN valve platform (Edwards Lifesciences, Irvine, CA, USA) is the commonest device used in the mitral VIV procedure8. The disadvantage of using a SAPIEN valve is the “one shot” procedure and hence the inability to reposition or retrieve the device after implantation if the above-mentioned complications are observed. To address these issues, and if necessary to remove the THV in case of concern with either coronary obstruction in an aortic VIV or LVOTO and inadequate fixation in the mitral VIV, a retrievable device is an attractive option over a non-retrievable device. Thus, one can implant the valve fully, check for the possibility of either of the issues, and if none then deploy the device, but if present either reposition it or retrieve it.
In their report, Schaefer et al describe a novel use of the Lotus™ valve (Boston Scientific, Marlborough, MA, USA) through a transapical approach to treat a degenerated Hancock II bioprosthesis (Medtronic, Minneapolis, MN, USA)9. The main highlight of this report is the ability to implant a THV and then to check for fixation and LVOTO before its release. This is indeed a step forward in the mitral VIV field and will have a positive impact on results and outcomes. The current limitations of the Lotus platform, however, are access (only the transapical approach is feasible) and sizes (the largest size is 27 as against 29 for the SAPIEN). These two limitations must be balanced against its advantages when choosing a Lotus over a SAPIEN valve.
The new tenth commandment for an ideal valve – “VIV proof design”
One of the ten commandments described by Dwight Harken was the ability to last lifelong1. Various attempts to find either an anticoagulation-free mechanical valve, newer anticoagulants with minimal side effects or newer synthetic or biological material have all failed to provide us with an “ideal valve”. VIV brings us closer to the holy grail. Increasingly, patients are choosing biological valves with the hope of VIV in the future, and hence the newer generation of surgical and THV designs must be modified to facilitate the VIV procedure.
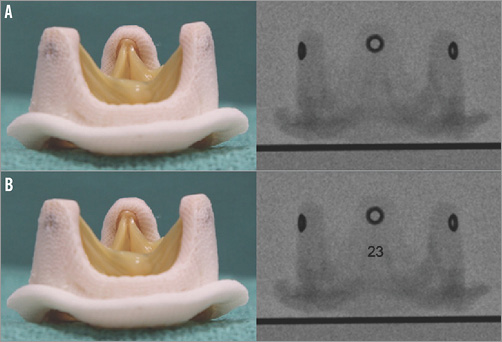
Thus, future valves (surgical and transcatheter) must be “VIV proof”. Features in surgical valve design, such as expandability to address the problem of treating small sizes in the aortic position and embedded fluoroscopic identification markers (Figure 1) to remove the confusion about sizing, will facilitate future VIV procedures by providing predictable results and eliminating complications. In the mitral position, design modification of the surgical valve to reduce the risk of LVOTO by altering leaflet height and projection into the left ventricle would be beneficial. Similarly, THV design modifications to accommodate VIV implant must be considered. One such feature is repositionability and retrievability, which was elegantly used by the authors in the report9.
Conflict of interest statement
V. Bapat is a consultant for Edwards Lifesciences, Medtronic and Boston Scientific.

