Abstract
Aims: To demonstrate the feasibility of the Leaflex™ Catheter System, a novel percutaneous device for fracturing valve calcification using mechanical impact in order to regain leaflet mobility.
Methods and results: Radiographic analysis of calcium patterns in 90 ex vivo human aortic valve leaflets demonstrated that 82% of leaflets had a typical “bridge” or “half-bridge” pattern, which formed the basis for the catheter design. The therapeutic effect was quantified in 13 leaflets showing a reduction of 49±16% in leaflet resistance to folding after treatment. A pulsatile flow simulator was then used with 11 ex vivo valves demonstrating an increase in aortic valve area of 35±12%. Using gross pathology and histology on fresh calcified leaflets, we then verified that mechanical impacts do not entail excessive risk of embolisation. In vivo safety and usability were then confirmed in the ovine model.
Conclusions: We demonstrated preclinically that it is feasible to improve valve function using the Leaflex™ technology. Once demonstrated clinically, such an approach may have an important role as preparation for or bridging to TAVI, as destination treatment for patients where TAVI is clinically or economically questionable and, in the future, maybe even as a means to slow disease progression in asymptomatic patients.
Introduction
Calcific degenerative aortic stenosis (AS) has become the most prevalent type of valvular heart disease in developed countries with ageing populations: it appears in about 2-7% of people 65 years and older. Calcification plays a major role in the formation and progression of the disease: starting at the microscopic level, calcific deposits within the valve leaflets and annulus grow into large calcium deposits that reduce leaflet mobility and thereby aortic valve opening area1,2.
Surgical replacement of the aortic valve, with meticulous manual removal of surrounding calcium deposits, remains the treatment of choice for most patients with symptomatic AS. For inoperable patients and those carrying high surgical risk, transcatheter aortic valve implantation (TAVI) is now the standard of care3-5 with proven haemodynamic improvement, but not without procedure complexity, high cost, and complications4,6,7. In contrast to surgery, TAVI does not attempt to address the native valve calcification, which probably accounts for most paravalvular leaks that affect therapeutic outcome, including higher long-term mortality8,9. Since the introduction of TAVI, the utilisation of balloon aortic valvuloplasty (BAV) has grown significantly4, but mainly as a means to dilate the native valve prior to crossing with the TAV prosthesis. The fundamental limitation of BAV as a potential destination therapy remains its inherent inability to affect valve calcification in a way that is meaningful enough to relieve symptoms beyond a very short period of time4,10-15.
Important proof that long-term calcium modification is indeed feasible and that re-calcification is a relatively slow process was provided decades ago by several surgical groups using ultrasonic and manual debridement16-20. It was shown that valve calcification could be effectively removed and that, when patients were followed up for relatively long periods of time, the incidence of “restenosis” (defined as the need for repeat surgery due to recurrence of symptoms) up to seven years following treatment was zero to 10%.
Encouraged by the potential durability of calcium modification, we sought to develop a device that would be designed to affect valve calcification in a safe, effective and durable way. We believe that such a non-implant-based technique, once demonstrated to be safe and effective, may offer a viable alternative to valve replacement in many patients, as well as a means to delay or prepare the “landing zone” for future valve implantation.
Our concept is based on fracturing of valve calcification using mechanical impact in order to regain leaflet mobility. The fractures created are internal and therefore do not expose the calcification to the blood stream, in order to minimise the risk of embolisation.
The Leaflex™ Catheter System
The Leaflex™ Catheter System (Pi-Cardia, Rehovot, Israel) (Figure 1) is a percutaneous device allowing delivery of controlled mechanical impacts to the aortic valve leaflets. These impacts are intended to fracture calcium deposits embedded within the leaflets focally, thereby restoring leaflet flexibility and mobility, achieving an increase in valve opening area.
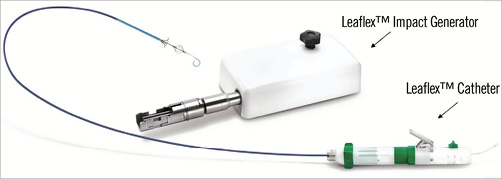
Figure 1. The Leaflex™ Catheter System.
The Leaflex is a disposable 13.2 Fr transfemoral catheter that includes two expandable nitinol elements, the Diamond and the Stabilizer (Figure 2), both connected by a unique set of shafts to an external Impact Generator. The Impact Generator receives compressed medical air in the cathlab and then transforms pneumatic energy into mechanical movements of the catheter shaft and Diamond.
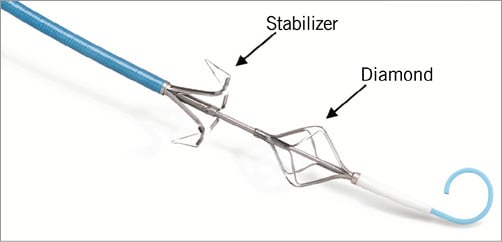
Figure 2. The Leaflex Stabilizer and Diamond.
The Leaflex mechanism of action is based on delivery of mechanical impacts to the valve: each impact is a rapid movement of the Diamond along a short distance towards the fixed Stabilizer. The Diamond and Stabilizer come in an expanded configuration (Figure 2) and are then sheathed into the catheter prior to delivery. The catheter is delivered to the aortic valve using the transfemoral approach. When across the valve, the Diamond is unsheathed, expanded and positioned on the ventricular side of the valve. The Stabilizer is then unsheathed and positioned on the aortic side in order to stabilise the device onto the valve leaflets. The two components are then brought together to “couple” the leaflets from both sides and an impact is delivered (Figure 3). The impact is generated in the Impact Generator using compressed air and transferred to the catheter as a mechanical movement. The compressed air is exhausted on the proximal side of the Impact Generator and does not enter the catheter or the patient. The rationale for positioning the impacting Diamond on the ventricular side of the valve was that the soft tissue covering the calcification on the ventricular side is typically thicker than on the aortic aspect2, and therefore remains intact and unharmed while the calcium deposits break. Valve coupling during treatment lasts only a few seconds, during which only partial occlusion occurs, and then the valve is released. This potentially obviates the need for rapid pacing. The short-amplitude movement of the Diamond against the fixed counteracting Stabilizer allows effective and controlled impacting and fracturing of calcium deposits without the risk of overstretching the annulus, tearing the leaflets or aorta, or “recoil” following treatment. The geometrical design of the Diamond and Stabilizer is aimed at optimising the location of fractures in the valve calcification.
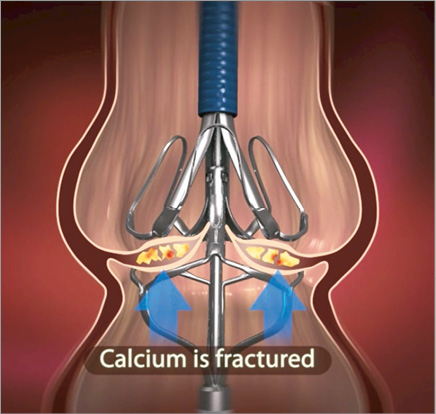
Figure 3. Stabilizer and Diamond couple the aortic valve.
We will now review the research done leading to the design and feasibility validation of the Leaflex catheter.
Identification of typical calcium patterns
Normal valve leaflets fold during systole, mainly along two folding lines, the attachment line of the leaflets to the annular base and the leaflet radial centreline (Figure 4)2,21. When calcium grows, this folding capability is sacrificed. Interestingly enough, calcium tends to grow in specific geometrical patterns21.
In order to characterise these patterns, we collected 90 surgically excised aortic valve leaflets from 30 patients who underwent surgical aortic valve replacement (SAVR). The leaflets were collected from five hospitals in Israel (Assuta Medical Center, Tel Aviv; Sheba Medical Center, Tel Hashomer; Soroka Medical Center, Beersheba; Herzliya Medical Center, Herzliya; Rambam Medical Center, Haifa). The study was approved by their ethics committees and patients signed informed consent forms (same protocol applies for all experiments described in this paper). Each valve leaflet was kept at 4°C, photographed and x-rayed using a Planmeca Prostyle Intra x-ray machine (Planmeca Oy, Helsinki, Finland). Calcium patterns seen in the x-ray images (Figure 5) were classified as either: 1) “bridge”, where calcification grows as two arcs from two sides of the leaflet base and then connects at the centre of the leaflet; 2) “half-bridge”, where the two arcs that grow from the leaflet base do not connect at the centre of the leaflet; or 3) “other” - all other patterns (Pi-Cardia, Rehovot, Israel, during 2011-2012).
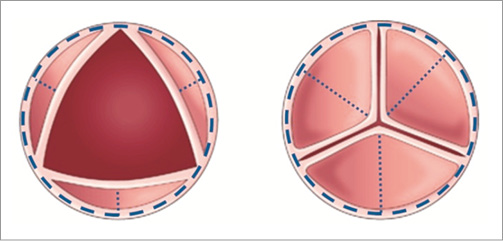
Figure 4. Main axes of leaflet movement during valve opening- annular base (dashed line) and central folding line (dotted lines).
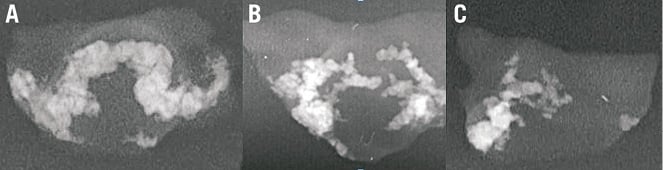
Figure 5. Calcium patterns: A) “bridge”; B) two “half-bridges”; C) “half-bridge”.
RESULTS
The majority of leaflets (82%) had either a “bridge” (47%) or “half-bridge” (35%) pattern. All patients had at least one leaflet with either a “bridge” or a “half-bridge”: 57% of patients had three leaflets with either a “bridge” or a “half-bridge”, 33% of patients had two leaflets with either a “bridge” or a “half-bridge”, and only 10% of patients had one leaflet with one of these patterns.
When calcium patterns cross a natural folding line of the leaflet, i.e., a “bridge” crossing both leaflet base and centreline or a “half-bridge” crossing the leaflet base, leaflet mobility is significantly sacrificed. We hypothesised that, by fracturing the “bridges” and “half-bridges” at these crossing points near the annulus and on the centreline, we could regain leaflet mobility (Figure 6).
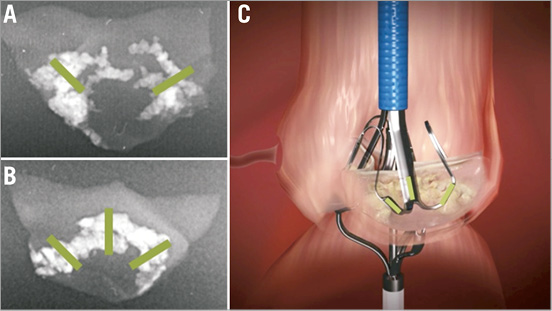
Figure 6. Optimal fracture locations on calcific deposits (A&B); Stabilizer footprint on leaflet (C).
Testing the effect of fractures on leaflet compliance
In order to demonstrate that fracturing calcification at the “hinge points” translates into increased leaflet mobility and compliance, we performed a series of mechanical impact tests on ex vivo human valve leaflets. Leaflet compliance before and after treatment was quantified by analysing force vs. displacement curves. Thirteen leaflets obtained from SAVR were glued to a silicone tube (mimicking the original annulus) along their original attachment lines in a typical anatomical position. Using a tensile machine, a cone was gradually displaced vertically, pressing against the leaflet from the ventricular aspect simulating ventricular blood pressure and forcing the leaflet to open (Figure 7). The forces required to achieve each leaflet displacement were measured to create force vs. displacement curves which were then used as a measure of leaflet compliance: the lower the force required for obtaining a certain cone/leaflet displacement, the more compliant the leaflet is (Figure 8). The evaluation of pre-treatment compliance of each leaflet was done twice in order to exclude set-up errors; the leaflets were then mechanically impacted in order to create fractures, and subsequently evaluated again (twice) for their post-treatment compliance (Pi-Cardia, Rehovot, Israel, during 2010).
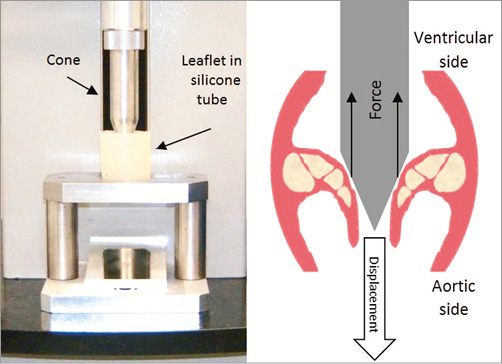
Figure 7. Test set-up for quantifying leaflet’s compliance.
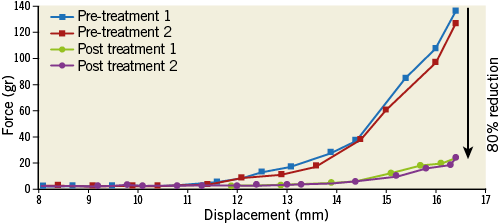
Figure 8. Force vs. displacement curve showing 80% reduction of the maximal force required to fold a calcified leaflet (at 16.5 mm displacement). Blue and red lines show two repeat measurements pre-treatment; green and purple lines show two repeat measurements post treatment.
RESULTS
In all leaflets, a reduction of more than 25% in the maximal force required to fold the leaflet was measured post treatment, with an average of 49±16%. In 54% of the leaflets the measured reduction was above 50%. These tests demonstrated that creating fractures in valve calcification was possible and translates into a significant increase of leaflet compliance.
Testing the effect of increased compliance on aortic valve area under flow
The next step was to demonstrate how increasing leaflet compliance by fracturing calcification using the Leaflex translates into increased aortic valve area. Since there is no large animal model for aortic stenosis22, we developed an ex vivo reconstructed valve model (Figure 9). The reconstructed valve is based on three calcified human valve leaflets removed during SAVR, sutured and glued in a three-dimensional coaptation position to a polyurethane component that has an anatomical shape of the aortic root.
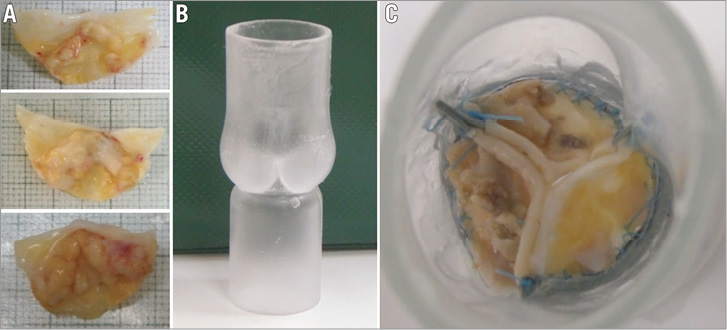
Figure 9. Calcific human aortic valve leaflets (A) and a polyurethane aortic root model (B) form the ex vivo reconstructed valve model (C - view from aortic side).
The reconstructed valve is then connected to a Pulse Duplicator (Pi-Cardia) (Figure 10), which simulates physiological pulsatile flow. The Pulse Duplicator allows full bench-top “catheterisation” of the reconstructed valve model using a model of the aorta (including the arch). The Pulse Duplicator enables measuring the geometrical aortic valve area (AVA) (Figure 11) by filming the functioning aortic valve from the ascending aorta over several heart cycles, capturing an image at peak systole and outlining the geometrical AVA by Digimizer™ software.
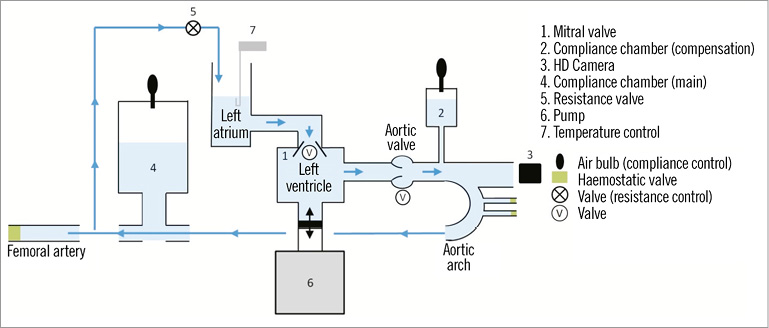
Figure 10. Pulse Duplicator set-up.
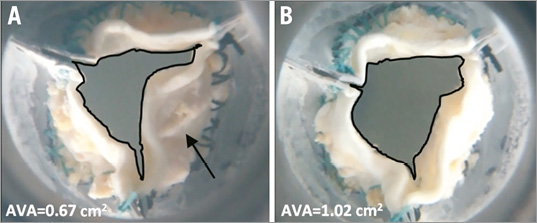
Figure 11. Ex vivo reconstructed valve opening area pre-treatment (A) and post treatment (B) as measured in the Pulse Duplicator set-up. Calcium deposit which prevented leaflet folding prior to treatment and then fractured by the Leaflex to improve AVA is marked by a black arrow.
Importantly, the significant limitations of measuring AVA using the reconstructed valve model are the following. 1) The leaflets, which are removed from the aorta during surgical AVR, are cut smaller than their full size and without the valve annulus. In the model, the missing annulus and leaflet-to-annulus attachment region are replaced by flexible latex and polyurethane materials. Consequently, some of the mechanical impacts delivered by the Leaflex with the intention of fracturing calcification actually impact the latex parts. In addition, the flexible latex region of the reconstructed valve affects the AVA both pre- and post-treatment. 2) During excision by the surgeon, the leaflets are frequently damaged and fractured even before treatment with the Leaflex, which affects the measured AVA pre-treatment. Despite these limitations, the reconstructed valve model is still a useful means to validate that fracturing calcification using the Leaflex produces an increase in AVA, even though absolute and percent change in AVA may not be indicative of what we should expect in patients. A relatively large number of reconstructed valve experiments were performed in order to develop and optimise the Leaflex. Eventually, 11 valves were used to perform the experiments with the final Leaflex design to test its effectiveness. In all reconstructed valves, x-ray images were used to record calcification patterns. AVA was measured in the Pulse Duplicator prior to treatment, five to 10 impacts were delivered, and AVA post treatment was measured. In addition, we looked at fractures created and at valve integrity post treatment (Pi-Cardia, Rehovot, Israel, during 2013).
RESULTS
1) In the 11 valves tested, all leaflets had a calcification pattern of either a “bridge” or a “half-bridge”: the majority (76%) of leaflets had a “bridge” pattern, 15% had one “half-bridge”, and the remaining leaflets (9%) had two “half-bridges”. 2) Fractures were created in all valves. 3) An average improvement in AVA of 35±12% was obtained. 4) In all experiments, valve integrity was maintained and good coaptation of the three leaflets during diastole was preserved. These results demonstrate that the Leaflex has the potential to increase the AVA in patients by fracturing calcium deposits without damaging the valve structure and integrity.
Human ex vivo safety tests of the Leaflex catheter
Having demonstrated that Leaflex increases AVA, it was critical to verify that the mechanical impacts did not entail excessive risk of embolisation. It is well known that valve interventions, such as SAVR, TAVI and BAV, all involve a non-negligible risk of embolisation23-27. Nevertheless, it was important to determine whether the interaction of Leaflex with valve tissue during treatment created an increased embolisation risk. It is important to emphasise, based on what we know from pathology, that valve calcification is covered by soft tissue on both ventricular and aortic aspects (Figure 12).
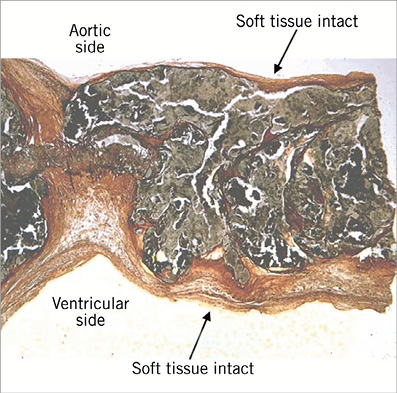
Figure 12. Leaflet histology (H&E and Weigert’s elastic stain) showing calcific deposit covered by a soft tissue on both ventricular and aortic aspects.
Accordingly, the mechanism of action and design of the Leaflex is based on fracturing calcium embedded within the leaflets without releasing it into the blood system. Fracturing is done focally with blunt Diamond struts on the ventricular side of the leaflets, where the tissue is thicker and more fibrous, while a counteracting force is applied by the Stabilizer on the aortic side. This mechanism is aimed at ensuring no injury to the soft covering tissue. Although no ideal model is available, in order to mitigate the risk of embolisation further, we performed an ex vivo test on fresh human calcified leaflets removed during SAVR. Two freshly excised valves were kept at 4°C for a few hours in order to make sure no deterioration of the tissue occurred. In each valve, two leaflets were glued to an artificial annulus and the third leaflet was used as control. The two leaflets were impacted five times by the Leaflex; the control leaflet was not treated. All leaflets were photographed and x-rayed pre- and post-treatment for gross pathology and sent for histopathology evaluation. Leaflets were put in formaldehyde immediately post treatment. To allow sectioning for histopathology evaluation (PathoVet, Rehovot, Israel) with minimal artefacts due to calcium deposits, the leaflets were placed in EDTA solution for 12 days prior to sectioning, in order to soften the calcium deposits (Pi-Cardia, Rehovot, Israel, during 2013).
RESULTS
Gross pathology showed that all treated leaflets remained intact on both the aortic and ventricular aspects (Figure 13), and were not different from the control leaflets. Histopathology further confirmed that the leaflets remained intact with no evidence of crush tissue necrosis, suggesting lack of severe or apparent damage by the Leaflex treatment.
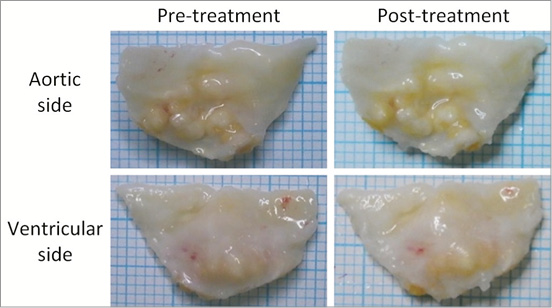
Figure 13. Pre- and post-treatment photographs of a treated leaflet.
In vivo animal testing of the Leaflex catheter
The final set of preclinical tests was performed in vivo on animals. The aim of the in vivo study was to evaluate the safety and usability of the Leaflex. We chose the ovine model as the preferred model for in vivo assessment of percutaneous aortic valve devices22,28. The model was used to validate the ability to deliver the catheter through the femoral arteries, over the aortic arch, through the aortic valve and into the left ventricle. However, the main limitation of any (large) animal model is that it does not have a diseased calcified aortic valve. Not only do the very thin healthy leaflets of the ovine aortic valve not provide any means for measuring therapeutic effect, but also critical parts of the Leaflex procedure such as device positioning and leaflet coupling could not be simulated in the animal model. Nevertheless, the ovine model was important for demonstrating the feasibility and usability of most procedure steps in a living animal, as well as ruling out potential complications, such as arterial/valve perforation, dissections and conduction system disturbance. Eight animals (female, Assaf breed) age two to four years and with a weight of 75-90 kg were included in the study, which took place in Sheba Medical Center in 2013 and was approved by their ethics committee.
To overcome the limitations of the ovine model and to test as many Leaflex procedure steps as possible, the safety and performance of the Leaflex were tested using two in vivo protocols, safety and performance. The safety aspect was tested in four animals by performing all procedure steps that do not rely on the calcified aortic valve, i.e., all steps excluding valve coupling and delivering impacts. The performance of the Leaflex was tested in four additional animals and included all procedure steps.
RESULTS
All steps of the Leaflex procedure were easily performed in all animals and showed: 1) easy catheter introduction and aortic arch and valve crossing over the guidewire; 2) easy Diamond and Stabilizer unsheathing and visualisation on fluoroscopy; 3) easy positioning of the Diamond and Stabilizer on the valve and impact delivery (performance protocol only); 4) easy Stabilizer and Diamond sheathing and catheter withdrawal (Figure 14). Post procedure, gross pathology and histopathology evaluations of the heart, the leaflets and the aortic arch were performed (PathoVet, Rehovot, Israel) in the four animals which underwent the safety protocol, and no major tissue damage was found.
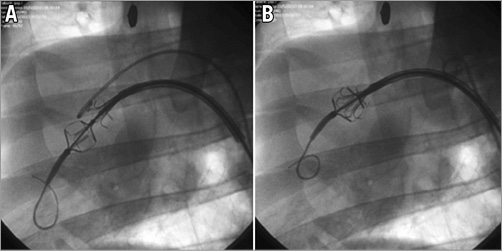
Figure 14. Leaflex in ovine model under fluoroscopy. Diamond and Stabilizer unsheathed (A) and coupled on the valve (B).
Discussion and summary
In this paper we describe a novel device and approach for treating the diseased aortic valve by directly addressing the underlying cause of the disease: valve calcification. The design of the Leaflex was based on extensive research into the typical calcium patterns and how they grow across the natural folding lines (“hinges”) of the leaflets, preventing them from folding properly2,21. Looking at early work done by several surgical groups16-20, we were encouraged to see that, if calcification could be carefully removed, patients could benefit from several years without recurrence of symptoms. Since, unlike during open surgery, it would be difficult and risky to attempt to remove calcification from the valve leaflets using a percutaneous approach, we were looking for a simple technique that would significantly modify valve calcification in order to restore leaflet mobility.
We then hypothesised that fracturing the calcific deposits at the “hinge points” would significantly increase leaflet compliance, as was nicely demonstrated by the force vs. displacement experiment. Using a reconstructed human valve model, we were then able to show that the increased leaflet mobility following treatment with the Leaflex translates into a significant increase in AVA. Based on pathological studies showing that valve calcification is covered by soft tissue2, we designed the Diamond and Stabilizer struts so that they were sufficiently blunt, and therefore impact was delivered in a way that did not injure the soft surrounding tissue in order to prevent embolisation. This key safety aspect was then demonstrated on freshly excised human leaflets.
The mechanism of action of the Leaflex is inherently different from that of BAV. Balloon valvuloplasty is based on dilating the valve, pushing valve leaflets and their embedded calcification radially outwards with little control over the outcome. For past aetiologies of rheumatic fever, where commissures were often fused, a balloon was an effective tool for tearing these apart, increasing the AVA. However, when leaflets are heavily calcified, there is very little control over the effect that BAV has on the valve: part of the radial force created by the balloon may be translated into uncontrolled, sometimes “open” fracturing of calcification, and part of it into dilation of the aortic root29. It is still difficult to know how much of the so-called BAV “restenosis” is due to fibrosis and re-calcification, as opposed to simple recoil of the aortic root. We hypothesised that, if significant and effective fractures could be created in a controlled fashion without dilating the aortic root, such as with the Leaflex, then the effect would be more durable than that observed following BAV.
With an ageing population, it is estimated that there are approximately 700,000 new cases of severe symptomatic AS every year. What are the treatment options for these patients? A recent poll in Europe estimated that around 63% of patients have their valve replaced by surgery. An additional 20% receive TAVI. For the remaining 17% there is very little that can be offered: some get a balloon (~4% of cases) and others, no treatment at all30.
It should not be surprising that, even with the excellent results of TAVI, about 45% of the high surgical risk patients in Europe still do not get their valve replaced30. TAVI remains a complex and costly procedure, and by definition many of these inoperable patients are extremely old, frail and sick. Currently, when the only relevant destination therapy is TAVI, the motivation is high to go ahead and implant a valve. We argue that, if a simpler, lower-cost alternative existed which could offer the patients at least one to two years without symptoms, such a technique could have an important role alongside TAVI.
In summary, we believe that a percutaneous device, such as Leaflex, once proven clinically safe and effective in modifying valve calcification, may have a number of important indications: 1) better preparation of the “landing zone” for TAVI; 2) bridging to TAVI or surgery; 3) destination therapy for patients where implanting a valve is questionable from a clinical or cost-effectiveness standpoint; and 4), looking into the future, perhaps even modifying the course of the disease in asymptomatic patients.
Study limitations
While the tests described in this paper demonstrate preclinical feasibility, our ability to predict the acute safety of the device in patients, such as risk for embolisation, acute efficacy and long-term durability of the treatment, is inherently limited. As there is no animal model for calcific AS, the safety, effectiveness and durability of the Leaflex treatment will ultimately have to be demonstrated in human clinical trials.
| Impact on daily practice The introduction of TAVI has revolutionised the field of structural heart disease. The natural expansion of TAVI into patients with lower surgical risk will further expose the significant patient population looking for catheter-based treatment of their valve disease. This growth also calls for the development of simpler, non-implant-based tools, such as Leaflex™, which can play an important role alongside TAVI: 1) offering a longer-term symptom relief than BAV in patients with significant comorbidities and short life expectancy, as an alternative or bridge-to TAVI; 2) preparing the “landing zone” in younger patients with bicuspid or heavy asymmetric calcification, in order to ensure good long-term outcome with TAVI; and finally 3) once Leaflex demonstrates clinical safety, effectiveness and long-term durability, it may offer a means to delay progression of the disease long before symptoms occur. |
Conflict of interest statement
M. Jonas, Y. Rozenman, Y. Moshkovitz, A. Hamdan, and E. Raanani are all consultants to Pi-Cardia. Y. Kislev, N. Tirosh, S. Sax, D. Trumer, and E. Golan are all employees at Pi-Cardia.

