
Abstract
Aims: The relevance of spontaneous echo contrast (SEC) and left atrial appendage thrombus (LAAT) in patients undergoing transcatheter aortic valve implantation (TAVI) remains unclear. In this study, we aimed to assess the prevalence of SEC and LAAT and evaluate the impact on periprocedural outcome after TAVI.
Methods and results: A total of 2,549 consecutive patients underwent TAVI between 2008 and 2017. After exclusion of cases with insufficient imaging, concomitant procedures or severe intraprocedural complications, 1,558 cases were analysed. Three groups were defined according to (pre)thrombotic formations – moderate or severe SEC (n=89), LAAT (n=53), and reference (n=1,416). The primary outcome was disabling ischaemic stroke within 24 hours. The prevalence was 4.4% for LAAT and 5.4% for moderate/severe SEC. The primary outcome occurred more frequently in patients with moderate/severe SEC (6.8%) compared to the reference (2.1%) and LAAT (1.9%) groups (p=0.020). SEC was identified as an independent risk factor for the primary outcome (OR 3.54 [95% CI: 1.30-9.61], p=0.013). LAAT was associated with an impaired unadjusted one-year survival (43.4%) compared to the SEC (27.3%) and reference groups (18.7%, p<0.001).
Conclusions: SEC and LAAT were detected in a relevant number of patients undergoing TAVI. SEC may represent an important risk factor for intraprocedural stroke; increased mortality was observed in patients with LAAT.
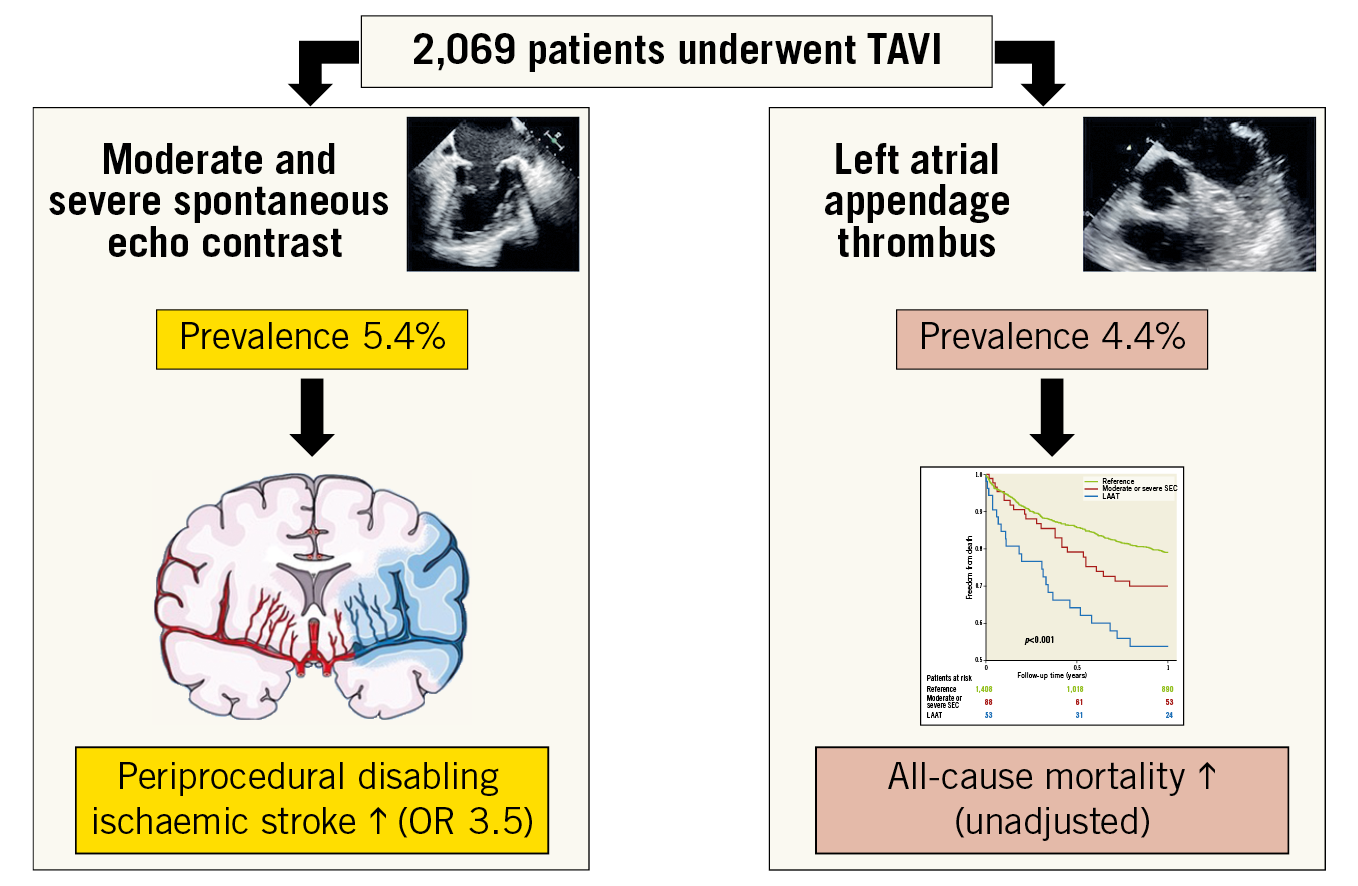
Visual summary. Left atrial appendage thrombus or (pre)thrombotic formations were observed in a relevant number of patients undergoing TAVI. An increased risk for periprocedural cerebrovascular events was observed if moderate or severe spontaneous echo contrast was present. The unadjusted all-cause mortality was higher in patients with left atrial appendage thrombus.
Introduction
Atrial fibrillation (AF) is a common comorbidity in patients undergoing transcatheter aortic valve implantation (TAVI). Cerebrovascular events remain a concern, even in low-risk patients1. Even though event rates are low (0.6-5.0%)1,2,3, stroke has a profound impact on morbidity, mortality, and quality of life after TAVI4,5.
Although the aetiology of stroke after TAVI is most likely multifactorial, an analysis of the early PARTNER trial demonstrated 51% of strokes to be procedure-related and 72% to be of ischaemic origin4. A recent analysis found that 48.9% of strokes occurred within 24 hours after TAVI and 68.4% within three days3, indicating the relevance of periprocedural embolisation. In addition to embolised aortic valve calcium or atheroma, thrombotic material from the left atrial appendage (left atrial appendage thrombus [LAAT]), or prethrombotic formations (spontaneous echo contrast [SEC]), may constitute a potential source for intraprocedural embolisation, particularly in patients with AF6,7. However, their exact prevalence and impact on intraprocedural stroke in larger patient samples undergoing TAVI remain unclear.
In this analysis we aimed to clarify the prevalence of SEC or LAAT in patients undergoing TAVI in a routine clinical setting and to evaluate their impact on periprocedural outcomes.
Methods
PATIENT POPULATION AND WORKUP
A total of 2,549 consecutive patients were treated with TAVI for severe symptomatic aortic stenosis at our institution during the period from 2008 to 2017. Patients with incomplete preprocedural transoesophageal echocardiography (TOE) imaging (n=480) were excluded from analysis, leaving 2,069 patients to be evaluated for the prevalence of LAAT and SEC (Figure 1).
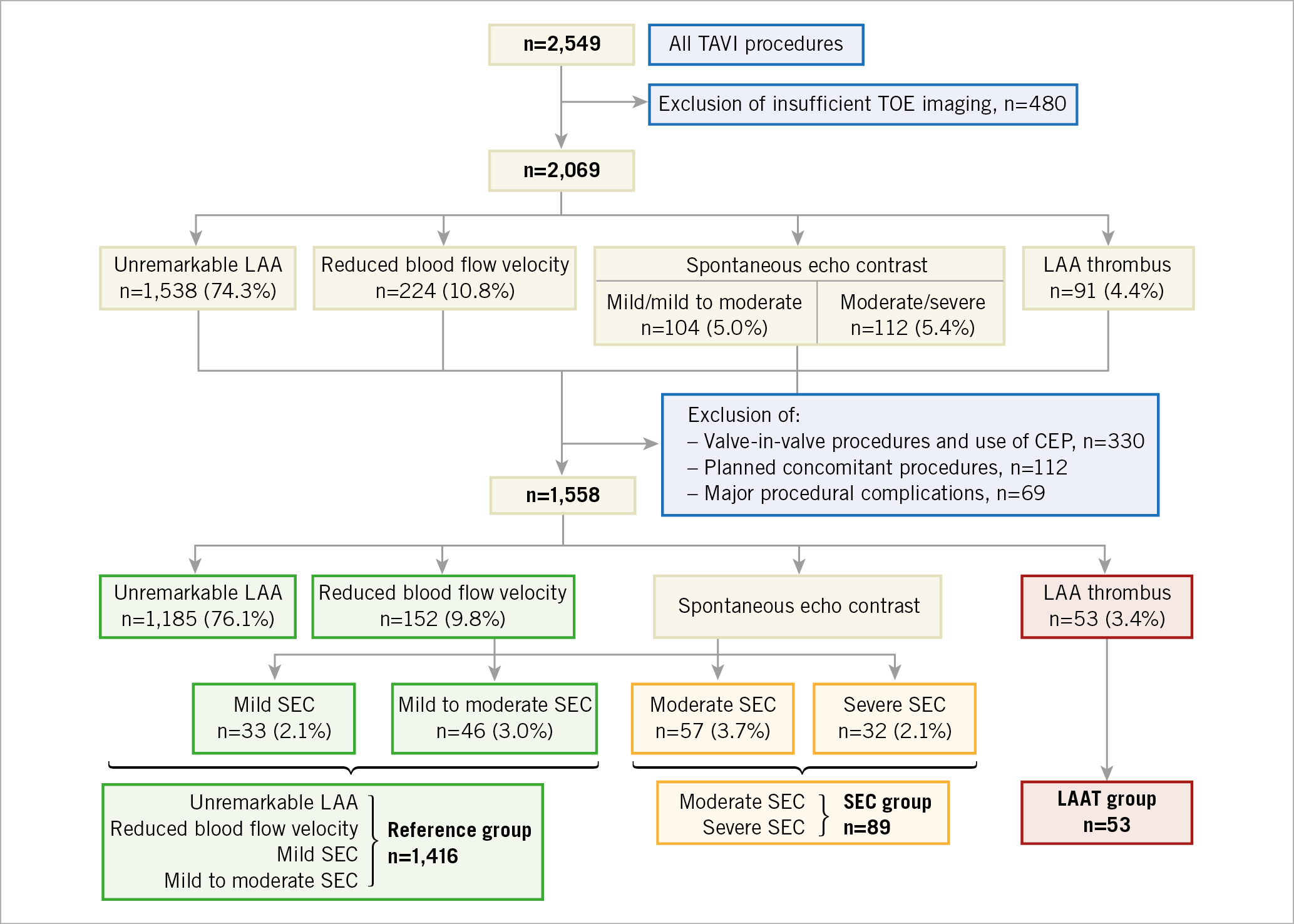
Figure 1. Patient population and allocation to study groups. CEP: cerebral embolic protection; LAA: left atrial appendage; LAAT: LAA thrombus; SEC: spontaneous echo contrast; TAVI: transcatheter aortic valve implantation; TOE: transoesophageal echocardiography
Outcomes were evaluated in 1,558 patients after exclusion of combined procedures (e.g., percutaneous coronary intervention or edge-to-edge mitral valve repair, n=112), procedures performed with cerebrovascular protection devices (CEP) or as valve-in-valve implantations (n=330) or with severe intraprocedural complications (e.g., aortic root rupture, cardiogenic shock, n=69) to minimise procedure-inherent confounding (Figure 1).
All cases were reviewed by the interdisciplinary Heart Team and were agreed to be eligible for a transcatheter approach. TAVI procedures were performed in a standard manner and under anticoagulation with unfractionated heparin to achieve an activated clotting time greater than 250 seconds throughout the procedure. After the procedure, anticoagulation with low-molecular-weight heparin was resumed in patients with indication for oral anticoagulation and switched to new direct thrombin inhibitors or phenprocoumon before discharge.
ECHOCARDIOGRAPHY
As part of routine clinical workup, both transthoracic echocardiography (TTE) and TOE were performed one to three days before the index procedure. All echocardiographic imaging data were evaluated retrospectively by two different experienced echocardiographers blinded to clinical outcome. Interobserver differences in the grading of SEC and the presence of LAAT were resolved by consensus. Findings in TOE were divided into four categories – unremarkable left atrial appendage (LAA), reduced blood flow velocity (<0.4 m/s), presence of SEC, and presence of LAAT. The severity of SEC was defined as described before8 (Supplementary Table 1).
Only patients with tricuspid aortic valves who suffered from severe aortic stenosis were included in this analysis. Evaluation of native aortic valve calcification was performed as described before9, using a semi-quantitative method.
DEFINITION OF STUDY GROUPS
Patients with unremarkable LAA (n=1,185), reduced blood flow velocity (n=152), mild (n=33) or mild to moderate (n=46) SEC were defined as the reference group. Patients with moderate (n=57) or severe (n=32) SEC were defined as the SEC group, and patients with a LAAT (n=53) were defined as the LAAT group (Figure 1).
STATISTICAL ANALYSIS
All relevant baseline, procedural, and imaging data were collected and entered into a dedicated database. Continuous variables were shown as median (interquartile range [IQR]) and binary variables as absolute numbers and percentages. They were compared with the Kruskal-Wallis test and with the χ² test, respectively. Survival curves for one-year all-cause mortality were produced using the Kaplan-Meier method. The log-rank test was used to test for survival curve differences. Adjusted logistic regression was performed to determine the association between the SEC group and the LAAT group, respectively, with the primary endpoint, compared to the reference group. We used a directed acyclic graph10 (Supplementary Figure 1) to evaluate the following potential confounding variables for the primary outcome – age, sex, prior stroke, peripheral artery disease, aortic valve calcification, history of AF, diabetes mellitus, renal function, impaired left heart failure, access route and transcatheter heart valve type. Following the results of the directed acyclic graph, logistic regression was adjusted for diabetes, glomerular filtration rate (GFR), AF, and left ventricular ejection fraction (LVEF) <30%. To account for the time of intervention, two subgroups (early group: 2008-2013 [n=847] and late group: 2014-2017 [n=711]) were defined and compared using an interaction analysis. All statistical analyses were performed using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
ENDPOINT DEFINITIONS
All endpoints were defined according to the updated Valve Academic Research Consortium-2 (VARC-2) criteria11. The primary endpoint was designed to measure the risk for peri-interventional stroke. Hence, it included disabling and ischaemic strokes that appeared within 24 hours after TAVI. Patients with new neurological deficits after TAVI were assessed by an experienced vascular neurologist. Symptoms and signs of <24 hours duration were defined as a transient ischaemic attack (TIA) and, if they persisted over 24 hours, they were classified as stroke11. The secondary endpoints included periprocedural complications.
Results
PREVALENCE OF LAA THROMBUS AND SPONTANEOUS ECHO CONTRAST
From all patients with sufficient TOE imaging (n=2,069) a solid LAAT was found in 4.4% and SEC in an additional 10.4% (moderate or severe SEC: 5.4%) (Figure 1).
In the patient population evaluated for the primary outcome (n=1,558), a solid LAAT was detected in 3.4% and moderate or severe SEC was found in 5.8% of patients (Figure 1, Supplementary Table 1). In cases with a known history of AF, the prevalence was 9.1% for LAAT and 25.7% for SEC (moderate or severe SEC: 14.6%). Thus, 23.7% of patients with AF were found with either LAAT or moderate/severe SEC, and 65.2% were without any SEC or LAAT.
PATIENT POPULATION
Compared to the reference group, we found a higher baseline risk profile in the SEC and LAAT groups including median age (81.4 years [IQR 76.8-84.9] vs 82.7 [IQR 78.3-85.6] vs 82.6 [IQR 78.4-85.1]; p=0.044), prior stroke (15.9% vs 29.2% vs 13.2%; p<0.0037), median glomerular filtration rate (57.5 ml/min [IQR 41.1-74.4] vs 52.8 ml/min [IQR 37.8-63.4] vs 49.5 [IQR 32.8-65.0]; p=0.0045) and history of AF (27.5% vs 82.0% vs 84.9%; p<0.001). Mean aortic transvalvular gradients at baseline were lower in the SEC and LAAT groups (32.0 mmHg [IQR 23.0-44.0] vs 28.0 [IQR 16.0-39.0] vs 28.0 [IQR 18.4-41.0]; p<0.001). The CHA2DS2-VASc score, the rate of peripheral arterial disease, diabetes, impaired LVEF, severe mitral regurgitation or pulmonary hypertension and calcification of the native aortic valve according to Rosenhek9 were similar among the groups (Table 1).
PROCEDURAL PARAMETERS
A non-transfemoral approach including general anaesthesia was selected in more patients with LAAT compared to the SEC and reference groups (63.8% vs 64.0% vs 41.5%; p=0.0043, and 68.9% vs 73.0% vs 84.9%; p=0.035) (Table 2). Selection of transcatheter heart valves (THV), predilatation and post-dilatation, need for rapid ventricular pacing and procedure times were similar. The volume of contrast agent was lower in LAAT patients compared to SEC and reference (150.0 ml [IQR 110.0-200.0] vs 147.0 ml [IQR 111.0-219.3] vs 126 ml [99.0-160.0]; p=0.016) (Table 2).

CLINICAL OUTCOMES
The primary endpoint of early disabling ischaemic stroke occurred more frequently in the SEC group than in the LAAT or reference groups (2.1% vs 6.8% vs 1.9%; p=0.020) (Table 3). A higher rate of overall early cerebrovascular events including strokes and TIAs (3.1% vs 7.9% vs 1.9%; p=0.044) and a trend to a higher incidence of disabling strokes at 30 days (2.5% vs 6.8% vs 1.9%; p=0.059) were observed in the SEC group. The incidence of haemorrhagic strokes was similar; the rate of all strokes and TIA at 30 days was 3.9% without significant differences (3.6% vs 7.9% vs 3.8%; p=0.13) (Table 4). All ischaemic strokes occurred within 24 hours after TAVI. All patients in the SEC and LAAT groups who suffered from cerebrovascular events were on therapeutic anticoagulation prior to the procedure. In the adjusted logistic regression, moderate or severe SEC was identified as an independent risk factor for disabling ischaemic stroke within 24 hours after TAVI (OR 3.54 [95% CI: 1.3-9.61]; p=0.013). LAAT did not demonstrate a significant association with the primary endpoint (OR 0.97 [95% CI: 0.12-7.64]; p=0.98) (Figure 2). Interaction analysis yielded no significant influence of the time of intervention on the primary outcome (early vs late groups, pinteraction=0.5). There were no differences in major access-site complications, major/life-threatening bleedings, myocardial infarctions, acute kidney injury stage 3 and VARC-defined device success. However, mortality at 30 days (4.5% vs 4.5% vs 15.1%; p=0.019), mortality at one year (20.96% vs 30.03% vs 46.18%; p<0.001) and the VARC-2 combined 30-day safety endpoint (13.6% vs 22.0% vs 32.05%; p<0.001) were particularly unfavourable for the LAAT group, with significantly increased event rates compared to SEC and reference patients (Table 4, Figure 3).
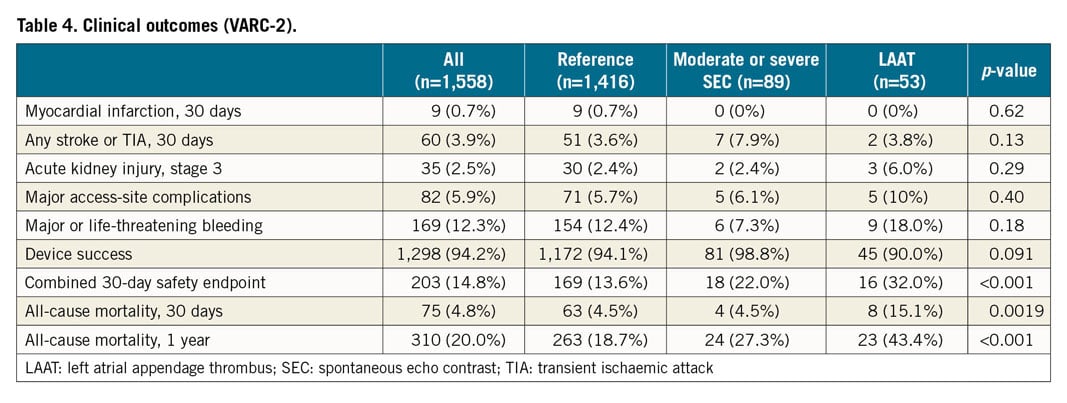
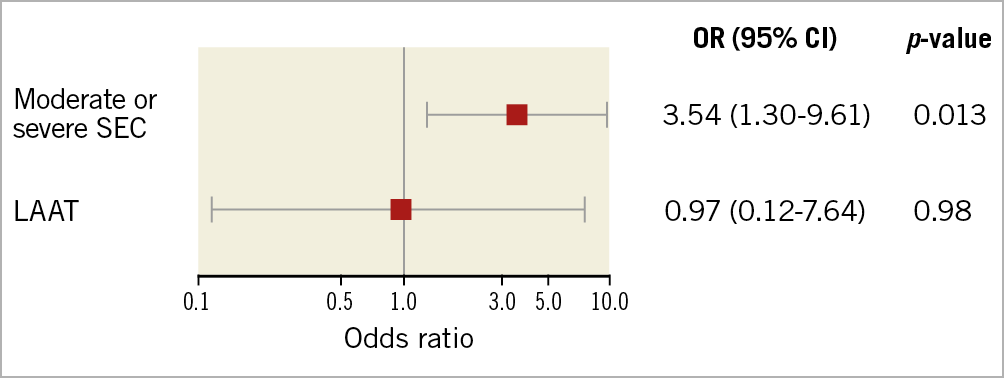
Figure 2. Forest plot for the primary endpoint (disabling, ischaemic stroke within 24 hours after TAVI) according to adjusted logistic regression analysis. LAAT: left atrial appendage thrombus; OR: odds ratio; SEC: spontaneous echo contrast
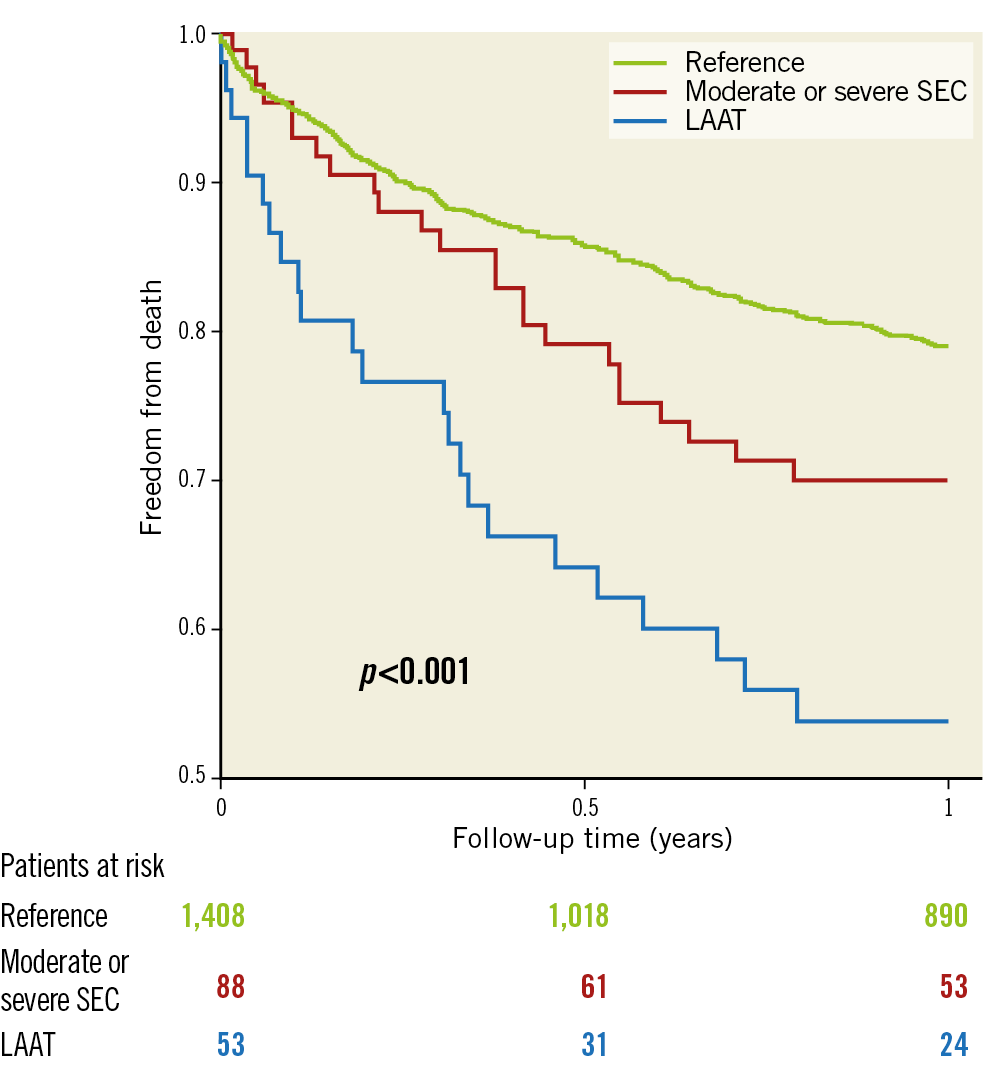
Figure 3. Unadjusted Kaplan-Meier survival curve at one year according to study groups. LAAT: left atrial appendage thrombus; SEC: spontaneous echo contrast
Discussion
Our analysis demonstrated (i) a relevant prevalence of LAAT and its precursors (moderate or severe SEC) in patients undergoing TAVI, (ii) a higher risk for intraprocedural strokes for patients with moderate or severe SEC, and (iii) a severely impaired survival in patients with LAAT.
PREVALENCE OF SEC AND LAAT
Previously, a small retrospective study detected LAAT according to delayed-phase computed tomography imaging in 11% of patients during TAVI workup12. We present the first analysis to investigate the prevalence of LAAT and SEC, as detected with TOE, in a large sample of patients undergoing TAVI in a routine clinical setting. As SEC was associated with stroke events previously7, we included these LAAT precursors in our analysis and found 9.8% of patients to have either moderate/severe SEC or LAAT. In patients with a known history of AF, moderate/severe SEC or LAAT was observed in as many as 23.7% of patients. The incidence of AF and the prevalence of LAAT were in line with previous publications on AF patients13. Interestingly, in the current analysis 16.9% of patients with LAAT or moderate/severe SEC were in sinus rhythm at admission and without any documented history of AF. Undetected paroxysmal AF may have led to the formation of LAAT or SEC. Nevertheless, none of these patients suffered a cerebrovascular event despite potentially insufficient anticoagulation. In addition to AF, predictors for SEC that have been reported before were age, increased filling pressures, low-flow states, left atrial size and mitral stenosis14.
CLINICAL OUTCOME
The study population reflects a high-risk cohort, covering almost 10 years and the early learning curve. This was emphasised by high rates of general anaesthesia, non-femoral access, and notable rates of access-site complications and mortality, if compared to current practice.
The main finding of the present analysis was a significantly higher incidence of intraprocedural ischaemic strokes in patients with relevant SEC, even after adjustment for risk factors and time of intervention. This matches well with previous reports on SEC and stroke risk7. The fact that all ischaemic events occurred within the first 24 hours after TAVI suggested an association with the procedure that requires further investigation. Cardioembolic origin seemed likely as typical brain imaging patterns were detected in all patients. Additionally, patients with severe intraprocedural haemodynamic compromise were excluded to rule out procedural malperfusion-induced cerebral ischaemia. SEC in the left atrium implied a low-flow state consistent with lower mean transvalvular gradients in SEC patients at baseline. Whether SEC itself caused cerebroembolism or served as a surrogate marker for other risks remains to be determined. Of note, prior strokes were particularly frequent in SEC patients.
In contrast to the above-mentioned results for SEC and a previous report12, we did not observe an increased stroke rate during TAVI in patients with LAAT. As SEC is considered a precursor of LAAT, this result remains unclear. LAAT was considered a contraindication for TAVI until resolution under sufficient anticoagulation had occurred. On rare occasions, TAVI was performed despite the presence of LAAT, e.g., for critical stenosis or after unsuccessful thrombus resolution. Whether intensified anticoagulation regimes may have positively influenced the risk for cardioembolic events in these cases will have to be investigated.
As opposed to our findings in early disabling ischaemic stroke, rates of all strokes at 30 days were not significantly different among groups, albeit they remained numerically higher in patients with moderate or severe SEC. This finding was driven mainly by numerically higher rates of non-disabling and haemorrhagic strokes in the LAAT and reference groups.
Unsurprisingly, unadjusted mortality was higher in patients with SEC and LAAT as they represent high-risk populations with severe comorbidities. However, a threefold increase in 30-day mortality in patients with LAAT compared to the SEC and reference groups despite similar intraprocedural complication rates raises concerns with regard to patient selection and management. SEC and LAAT may serve as surrogate markers of individual risk rather than independent risk factors for mortality based on the present observation. For this reason, we deliberately waived multivariate analysis for mortality.
PREVENTIVE MEASURES
Due to a suspected multifactorial aetiology of strokes after TAVI, general measures of prevention should be undertaken.
First, identification of patients at risk remains essential. As suggested by our results, TOE is a valuable tool to identify these patients. However, it has been omitted from routine workup before TAVI in most centres in order to streamline processes. Due to its high sensitivity for the detection of LAAT or SEC12, delayed phase ECG-gated computed tomography may allow sufficient diagnosis, leaving TOE for ambiguous cases. Computed tomography protocols used for routine pre-TAVI workup may have to be adjusted, however, for proper collection of the required information. The fact that a relevant number of supposedly non-AF patients were found with LAAT or SEC underlines the need for careful evaluation of all TAVI patients irrespective of their medical record. Atrial cardiopathy and dysfunction may modulate stroke risk even in patients without atrial fibrillation15. Additional risk factors include patent foramen ovale, aortic atheroma, left ventricular thrombus and undocumented AF16,17,18.
Second, sufficient anticoagulation is important and cardioembolic protection may be sensible. It is common practice in patients with LAAT to intensify anticoagulation and postpone TAVI until resolution of thrombus has occurred. Additionally, and particularly in urgent cases which cannot be postponed, the use of CEP during TAVI may alleviate the intraprocedural embolic stroke risk. A reduction of the median number and volume of new brain lesions in magnetic resonance imaging was demonstrated19, and a retrospective analysis suggested a reduction of intraprocedural strokes with CEP2. Considering the associated stroke risk with SEC, CEP may be reasonable in these patients at risk. In addition, uninterrupted periprocedural anticoagulation and omission of heparin reversal with protamine at the end of the procedure may be appropriate in patients with high ischaemic and moderate bleeding risk. We usually interrupt anticoagulation on the day of TAVI to minimise periprocedural bleeding but this practice may need to be re-evaluated in patients with a predominant ischaemic risk. Whether avoidance of intraprocedural low-flow states by omission of rapid ventricular pacing may be beneficial needs further investigation.
Limitations
Limitations of the current analysis relate to the retrospective observational study design, low event rates for the primary outcome and differences in group sizes. Statistical methods were selected to minimise these limitations. We adjusted for typical risk factors to evaluate the association of SEC and LAAT with the primary outcome. However, not all confounding variables may have been accounted for sufficiently. Although higher rates of early ischaemic strokes were observed in patients with SEC, this was not found for LAAT, something which remains unexplained. Overall, LAAT or SEC may serve as surrogate parameters for confounding risks that remained unknown at analysis and for which no causal relationship can be established. To gain sufficient patient numbers, procedures from 2008 to 2017 were analysed, which also included the early learning curve. This is illustrated by high rates of general anaesthesia, transapical access and notable rates of short-term and midterm mortality compared to current practice. These aspects should be kept in mind when applying the results of this analysis to current TAVI patient populations.
Conclusions
This retrospective analysis demonstrated that a relevant number of patients who underwent TAVI in clinical routine had SEC or LAAT. As SEC in particular was strongly associated with more intraprocedural disabling ischaemic strokes and increased mortality was observed in patients with LAAT, both will help to identify patients at particular risk. The initiation of preventive measures will be of particular importance to improve outcomes after TAVI further.
|
Impact on daily practice Spontaneous echo contrast (SEC) or left atrial appendage thrombus (LAAT) was detected in a relevant number of patients scheduled for transcatheter aortic valve implantation. SEC may represent an important risk factor for intraprocedural stroke; increased mortality was observed in patients with LAAT. These patients at risk need to be identified beforehand to tailor therapeutic strategies and evaluate preventive measures in order to improve overall outcomes. |
Conflict of interest statement
M. Linder, L. Voigtländer and O.D. Bhadra have received travel compensation from Edwards Lifesciences. M. Seiffert served as consultant for JenaValve and Boston Scientific, has received travel compensation from Edwards Lifesciences, JenaValve, Boston Scientific and Biotronik, and has received speaker honoraria from Medtronic. N. Schofer has received travel compensation from Edwards Lifesciences and St. Jude Medical, as well as speaker honoraria and travel compensation from Boston Scientific. J. Schirmer is a proctor for Boston Scientific and JenaValve. U. Schäfer is a consultant and proctor for Edwards Lifesciences, Abbott, Biotronik, JenaValve, Medtronic, New Valve Technology and Boston Scientific, and has received speaker honoraria and travel compensation from JenaValve, Edwards Lifesciences, Abbott, Medtronic, New Valve Technology and Boston Scientific. L. Conradi is a proctor for and has received speaker honoraria as well as travel compensation from JenaValve, Edwards Lifesciences and Boston Scientific, has received speaker honoraria and travel compensation from Medtronic, and is a consultant for Edwards Lifesciences. D. Westermann has received honoraria from AstraZeneca, Bayer, Böhringer-Ingelheim, Berlin Chemie, Novartis and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

