
Disabling stroke is the complication patients fear most when discussing treatment of severe aortic stenosis. The reported incidence of stroke following transcatheter aortic valve implantation (TAVI) varies from 1 to 9%: the PARTNER 3 trial demonstrated a 30-day stroke rate of 0.6% in low-risk patients, and the SENTINEL trial demonstrated a 9% stroke rate in the control group of patients1,2. As the threshold for considering TAVI moves into a younger and lower-risk population, the impact of disabling stroke on morbidity, mortality and healthcare costs becomes even more relevant.
Patient and procedural factors contribute to the risk of stroke early after TAVI and include conventional risk factors for stroke including atrial fibrillation (AF), peripheral vascular disease, patient age, and diabetes, whilst other factors are unique to the TAVI procedure such as predilatation or post-dilation or valve repositioning3. Although clinical scores for predicting stroke risk in AF are well established, we are not yet in a position to estimate stroke risk for individual patients being considered for TAVI in order to tailor the decision making and personalise the procedure.
In this edition of EuroIntervention, the analysis by Linder et al of a large retrospective observational consecutive cohort study provides new data on the impact of spontaneous echo contrast (SEC) and left atrial appendage thrombus (LAAT), documented by routine transoesophageal echocardiography (TOE), on stroke and mortality following TAVI4.
SEC - also often termed “smoke” – is an abnormality noted during echocardiography and caused by aggregates of red blood cells occurring in low flow and low shear conditions. Its association with thromboembolic events is well established in patients with AF, increasing the stroke rate nearly fourfold. LAAT is the consequence of coagulation of blood in very slow flow or stasis within the appendage. It is thought to be the origin of cardioemboli in ~90% of patients with AF and stroke or transient ischaemic attack (TIA) (Figure 1).
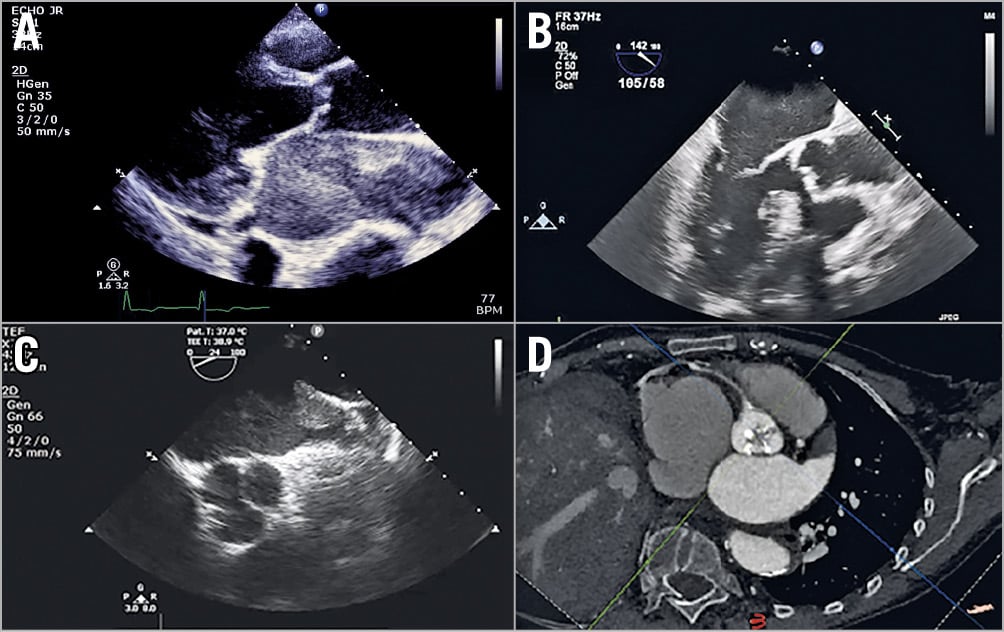
Figure 1. Examples of spontaneous echo contrast and thrombus in the left atrium. A) Dense spontaneous echo contrast seen on a transthoracic echo in a patient with severe rheumatic mitral and aortic stenosis. B) Mild spontaneous echo contrast seen on a transoesophageal echo in a patient with severe degenerate aortic stenosis. C) Large thrombus in mouth of left atrial appendage seen on a transoesaphogeal echo. D) Cardiac CT reconstructed to demonstrate calcified stenotic aortic valve and large thrombus in left atrial appendage.
In the 2,069 patients in this study, 206 showed SEC (112 had moderate/severe SEC), and 91 patients had solid LAAT. Clinical outcome was assessed in 1,558 patients and compared patients with moderate/severe SEC (n=89) and LAAT (n=53) with a reference group of 1,416. After risk adjustment, SEC was independently associated with an incidence of stroke of 6.8%, versus 2.1% in the reference group, representing a threefold increased risk of stroke within 24 hours of TAVI. Thirty-day mortality was significantly higher in the LAAT group and both the SEC and LAAT groups had higher mortality than the reference group at 12 months. Other groups have also reported on the impact of LAAT, with variable findings5,6. Do these data further inform our understanding of stroke in TAVI and help us to predict patients at high stroke risk?
Although the vast majority of patients who had SEC also had AF, only around one in four patients with AF had SEC or LAAT. Identifying the subset of AF patients at higher risk would be an important step forward to identify those at highest stroke risk. Most established TAVI programmes have moved to preprocedural planning with cardiac computed tomography (CT) rather than TOE, given the ability to evaluate the aortic root, valve, LVOT anatomy, access vessels and coronary arteries in one non-invasive low-risk study. Evaluating the LAA for thrombus with delayed contrast-enhanced CT appears to be at least equivalent to TOE7, and acquisition techniques to identify and quantify slow flow in the left atrium are being developed and evaluated8. It would seem a retrograde step to return to a preprocedural TOE in all patients being considered for TAVI; perhaps instead we should extend our CT analysis to include the left atrium and its appendage.
The second question of how we might attenuate stroke risk remains unanswered. In this cohort, patients who were anticoagulated had this discontinued for the TAVI procedure, as is routine practice, and patients who had received a cerebral embolic protection (CEP) device were excluded from the analysis. Thus, rather than using a standard procedural protocol of heparinisation and protamine reversal, should we identify patients who may be at higher risk of thromboembolic stroke and tailor their anticoagulation strategy appropriately? Proceeding without interruption of anticoagulation or avoidance of complete reversal of heparinisation once vascular closure is secure are options which need further study. In the group with LAAT, the apparent policy of not proceeding with TAVI unless critically urgent and instead intensifying anticoagulation and ensuring that clot resolution has occurred may explain the lower stroke risk and suggest an alternative approach which needs to be evaluated. Furthermore, although CEP seems to reduce the number and volume of new lesions on neuroimaging, and may reduce the rate of stroke and death, randomised controlled trials remain ongoing9. It is possible that CEP may be more effective in high-risk groups, such as defined by this study.
As personalised and precision medicine become established approaches, it is increasingly important that we provide a patient-relevant estimation of risk wherever possible. Preprocedural imaging helps us to tailor the prosthesis type and size and guide the vascular access strategy. If we include assessment of thrombosis risk in the left atrium and appendage, we may be able to refine periprocedural anticoagulation strategies or the use of CEP in order to reduce the incidence of stroke, and thus improve the outcomes for patients after TAVI.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

