Abstract
BACKGROUND: Severe degenerative mitral regurgitation (DMR) can cause a poor prognosis if left untreated. For patients considered at prohibitive surgical risk, transcatheter edge-to-edge repair (TEER) has become an accepted alternative therapy. The DragonFly transcatheter valve repair system is an innovative evolution of the mitral TEER device family to treat DMR.
AIMS: Herein we report on the DRAGONFLY-DMR trial (ClinicalTrials.gov: NCT04734756), which was a prospective, single-arm, multicentre study on the safety and effectiveness of the DragonFly system.
METHODS: A total of 120 eligible patients with prohibitive surgical risk and DMR ≥3+ were screened by a central eligibility committee for enrolment. The study utilised an independent echocardiography core laboratory and clinical event committee. The primary endpoint was the clinical success rate, which measured freedom from all-cause mortality, mitral valve reintervention, and mitral regurgitation (MR) >2+ at 1-year follow-up.
RESULTS: At 1 year, the trial successfully achieved its prespecified primary efficacy endpoint, with a clinical success rate of 87.5% (95% confidence interval: 80.1-92.3%). The rates of major adverse events, all-cause mortality, mitral valve reintervention, and heart failure hospitalisation were 9.0%, 5.0%, 0.8%, and 3.4%, respectively. MR ≤2+ was 90.4% at 1 month and 92.0% at 1 year. Over time, left ventricular reverse remodelling was observed (p<0.05), along with significant improvements in the patients’ functional and quality-of-life outcomes, shown by an increase in the New York Heart Association Class I/II from 32.4% at baseline to 93.6% at 12 months (p<0.001) and increased Kansas City Cardiomyopathy Questionnaire (KCCQ) score of 31.1±18.2 from baseline to 12 months (p<0.001).
CONCLUSIONS: The DRAGONFLY-DMR trial contributes to increasing evidence supporting the safety and efficacy of TEER therapy, specifically the DragonFly system, for treating patients with chronic symptomatic DMR 3+ to 4+ at prohibitive surgical risk.
Mitral regurgitation (MR) is the most prevalent heart valve disease, particularly in populations aged >75 years1. MR increases left atrial and pulmonary venous pressure, leading to symptoms such as fatigue and dyspnoea2. Untreated severe MR is associated with pulmonary hypertension, atrial fibrillation, heart failure (HF), and mortality3. One of the more common aetiologies of MR is degenerative mitral regurgitation (DMR) which involves abnormities of the mitral valve, and in DMR, medical therapy does not improve survival4. Surgical mitral valve repair, with proven efficacy and a well-established safety profile, is a Class I recommendation in the current guidelines for symptomatic patients with DMR56. Nevertheless, owing to the perception of prohibitive surgical risk, aversion to surgery, or comorbid conditions78, there remains an ongoing need for less invasive treatment options with the development of new technology and devices.
Transcatheter edge-to-edge repair (TEER) is an increasingly acknowledged treatment for patients with DMR who have a prohibitive surgical risk. The EVEREST II trial (Endovascular Valve Edge-to-Edge Repair Study) of the MitraClip (Abbott) demonstrated that TEER is a safe and effective therapy for these patients910. The MitraClip (now in its fourth generation) has been the mainstay of TEER therapy, used in over 150,000 patients worldwide. The PASCAL system (Edwards Lifesciences) was introduced more recently as another TEER therapy, and CLASP IID (Edwards PASCAL TrAnScatheter Valve RePair System Pivotal Clinical Trial) demonstrated its safety and effectiveness, which were comparable to those of the MitraClip11. With the accumulation of clinical evidence, recent guidelines from the American Heart Association/American College of Cardiology and European Society of Cardiology/European Association of Cardio-Thoracic Surgery have recommended that in symptomatic patients with severe DMR and high or prohibitive surgical risk, TEER can be appropriate56.
The DragonFly transcatheter valve repair system (Valgen MedTech) is similar in concept to the MitraClip and PASCAL systems but has distinguishing and unique features. The system was initially studied in the first-in-human Dragonfly-M Early Feasibility Study, in which TEER using the DragonFly system was demonstrated to be feasible and safe for the treatment of patients with severe MR12. Herein, the DRAGONFLY-DMR trial aimed to further evaluate the safety and effectiveness of the DragonFly system for patients with symptomatic (moderate-severe and severe) DMR who are considered to be at high surgical risk and whose mitral anatomy is suitable for the TEER procedure.
Methods
STUDY DESIGN AND POPULATION
This prospective, multicentre, single-arm study was conducted at 27 sites in China. Study inclusion criteria required that all patients be symptomatic with chronic moderate-to-severe (3+) or severe (4+) DMR and were assessed as having at least a high risk for surgical mitral repair by the cardiac team at the local clinical trial site. Patients were also required to meet the following criteria: age ≥18 years; New York Heart Association (NYHA) Functional Class II, III, or IV; left ventricular ejection fraction ≥20%; anatomically suitable for mitral valve repair with the DragonFly device; and at least a high surgical risk. High surgical risk was defined per the recommended reference criteria, as follows: surgical valve replacement Society of Thoracic Surgeons (STS) score of ≥8; surgical valve repair STS score of ≥6; or the presence of other surgical high-risk factors, such as the ≥2 moderate-to-severe indicators of frailty, surgery-specific impediments (including tracheostomy, heavily calcified [porcelain] ascending aorta, and chest malformation) according to the guidelines for management of valvular heart diseases, or the presence of ≥2 major organ dysfunction that cannot be improved in the postoperative period or other surgical high-risk factors, as judged by the cardiac team513.
Patients were excluded if they had echocardiographic evidence of an intracardiac mass, thrombus, or vegetation; the presence of other severe non-mitral valve disease requiring intervention; history of previous mitral valve surgery or transcatheter mitral valve intervention; severe pulmonary arterial hypertension (pulmonary artery systolic pressure [PASP] >70 mmHg); history of acute myocardial infarction within 4 weeks; untreated severe coronary artery stenosis requiring revascularisation; any cardiovascular interventional procedure within 30 days; or any cardiac surgical procedure performed within 6 months. The complete inclusion and exclusion criteria are shown in Supplementary Table 1. Note that the determination of anatomical suitability was adjudicated by two independent experienced TEER operators on the eligibility committee.
The study protocol was designed following the guidelines of the Mitral Valve Academic Research Consortium; it was approved by the investigational review board/ethics committee at each participating site and conducted according to the principles of the Declaration of Helsinki. All patients provided written informed consent, and the study conformed to the Good Clinical Practice principles and ISO 14155:2020. Echocardiographic images were evaluated by an independent echocardiography core laboratory. A clinical event committee (CEC) adjudicated prespecified major adverse events (MAE). The sponsor participated in the site selection, trial management, and data analysis; however, all patients’ study eligibility was determined by each site's Heart Team and confirmed by the independent eligibility committee.
After signing an informed consent form, participants were enrolled and treated using the DragonFly System (DragonFly transcatheter mitral valve repair system). Follow-up study visits were conducted immediately after the procedure, before discharge, and at 30 days, 6 months, and 12 months after the procedure.
This study is registered at ClinicalTrials.gov (NCT04734756; Safety and Effectiveness Study of Dragonfly System for Degenerative Mitral Regurgitation) and sponsored by Valgen Medtech. Trial organisation, leadership and participating sites are listed in Supplementary Table 2 and Supplementary Table 3.
THE DRAGONFLY TRANSCATHETER MITRAL VALVE REPAIR SYSTEM AND PROCEDURAL DETAILS
The DragonFly system comprises four components: a stabiliser, a 24 Fr guiding sheath (big sheath), a steerable sheath (middle sheath), and a device delivery system (control handle), with the DragonFly implant preattached at the end. Four implant sizes are available for application in different anatomical conditions (Central illustration A)
DragonFly has a compressible atrial-side central filler and a mechanically locked arm angle between 0° and 45°. As the arms are closed around the compressible filler, the filler distends on either side of the device, further blocking the regurgitant orifice. Control of the device’s final arm angle allows for individual adjustment relative to the mitral valve orifice area. Additionally, the narrow design of the arms allows for placement amidst dense chordae, such as in the commissures. Additionally, the mechanical locking force of the device allows for sufficient clamping force to address cases with degrees of leaflet calcification at the device placement site. The implantation procedure has been previously described1314 and is outlined in Supplementary Appendix 1.
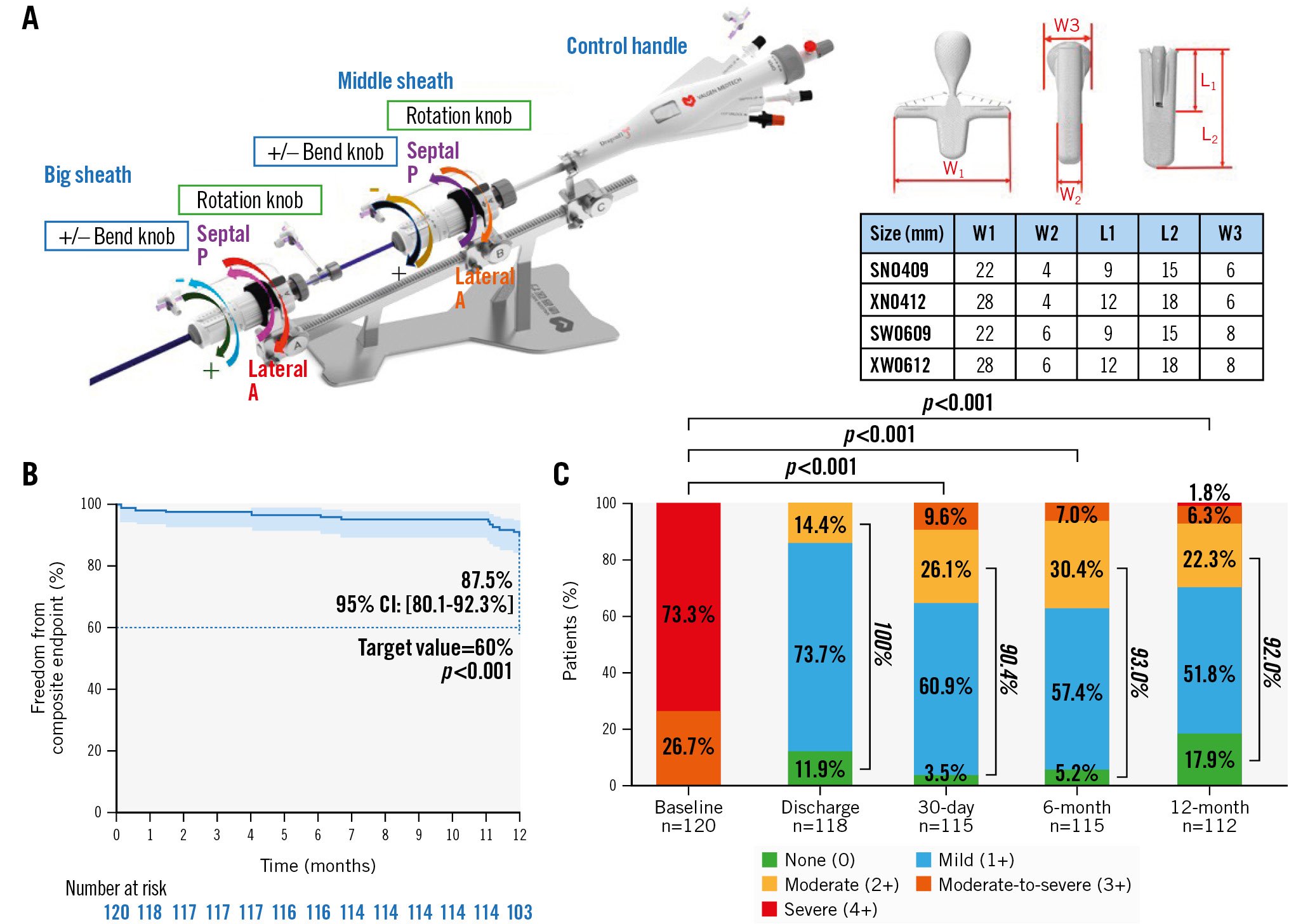
Central illustration. The DragonFly transcatheter mitral valve repair system with 1-year outcomes. A) DragonFly transcatheter mitral valve repair system design and device implantation parameters. The four clips are suitable for diverse anatomical conditions. B) Kaplan-Meier estimates for the primary composite endpoints. The error bars represent 95% CI. The primary composite endpoints included freedom from death, mitral valve-related reintervention due to mitral valve dysfunction, and moderate-to-severe or severe mitral regurgitation (MR) >2+ at 12 months. MR >2+ was determined based on the 12-month transthoracic echocardiography with echocardiography core laboratory confirmation. C) MR severity assessed by the echocardiography core laboratory using transthoracic echocardiography. The graph shows unpaired analyses, and p-values were calculated using the Wilcoxon signed-rank test. CI: confidence interval; L1: length of the arms; L2: length of the clip; N: narrow; S: short; W: wide; W1: width of arm opening at 180°; W2: width of the clip; W3: width of central compressible filler; X: extra
ENDPOINTS
The study’s primary efficacy endpoint was clinical success at 12 months, which was defined as freedom from mortality, reintervention for mitral valve dysfunction, and moderate-to-severe or severe MR >2+. Secondary efficacy endpoints included acute procedural success (defined as the successful implantation of the device with MR ≤2+ at discharge), acute device implantation success (defined as the successful delivery and deployment of one or more devices, with echocardiography confirming secure leaflet insertion, and successful retrieval of the delivery catheter), reintervention due to mitral valve dysfunction, NYHA classification, and Kansas City Cardiomyopathy Questionnaire (KCCQ) scores. Safety endpoints included MAE (defined as procedure-related mortality, stroke, myocardial infarction, renal failure, and cardiovascular reintervention related to the procedure or device), all-cause mortality, and cardiac mortality. Heart failure hospitalisation (HFH) was an extended observational endpoint.
ECHOCARDIOGRAPHIC ASSESSMENTS
Image acquisition was performed following the echocardiography core lab (ECL)-recommended protocol. All echocardiograms obtained at baseline, discharge, and follow-ups were assessed by the ECL according to pre-established protocols based on the American Society of Echocardiography guidelines. MR severity was graded on a scale of 0 to 4+1516. Transthoracic echocardiography (TTE) or transoesophageal echocardiography (TOE) was utilised for baseline qualification, procedural planning, and intraprocedural imaging guidance, and TTE was used for follow-up assessments.
STATISTICAL ANALYSIS
All analyses were performed using the full analysis set. Continuous variables are summarised as the number of observations, mean±standard deviation (SD) or median (interquartile range: first quartile [Q1]-third quartile [Q3]), and 95% confidence interval (CI). The p-values for continuous variables were calculated using the Student’s t-test. The paired analysis comprised data for the same patient during a specified follow-up. McNemar’s test was used to assess binary repeated measures. Categorical variables are summarised as patient count, percentage, and 95% CI and were compared using the Wilcoxon signed-rank test. Kaplan-Meier estimates were used to analyse the time-to-event variables. Unless otherwise stated, patients with missing data were excluded from the denominator. Statistical analyses were performed using SAS software version 9.4 (SAS Institute).
Results
PATIENTS
A total of 120 patients from 27 sites in China were enrolled and treated between May 2021 and January 2022. The final follow-up was completed in December 2022. Of the 120 patients, one did not receive the device and, thus, was not included in the per-protocol analysis but was included in the full analysis set (Figure 1). The mean age of the participants was 74.9±5.7 years, with 49.2% (59/120) being female. Overall, 39.2% (47/120) had coronary heart disease, 18.3% (22/120) had a prior history of cardiovascular intervention or surgery, and 70.8% (85/120) had chronic obstructive pulmonary disease. The mean STS score for replacement (version 4.20) was 6.9±2.8. In total, 65.9% (79/120) of patients were in NYHA Functional Class ≥III (Table 1). All patients had MR grade ≥3+, with 73.3% (88/120) having MR grade >4+; 55.8% (67/120) of patients had prolapse involving the P2 area, whereas 13.3% (16/120) had prolapse in the A2 area (Table 2). Moreover, the presence of ≥2 moderate-to-severe indicators of frailty was the most common reason for prohibitive risk (Supplementary Table 4).
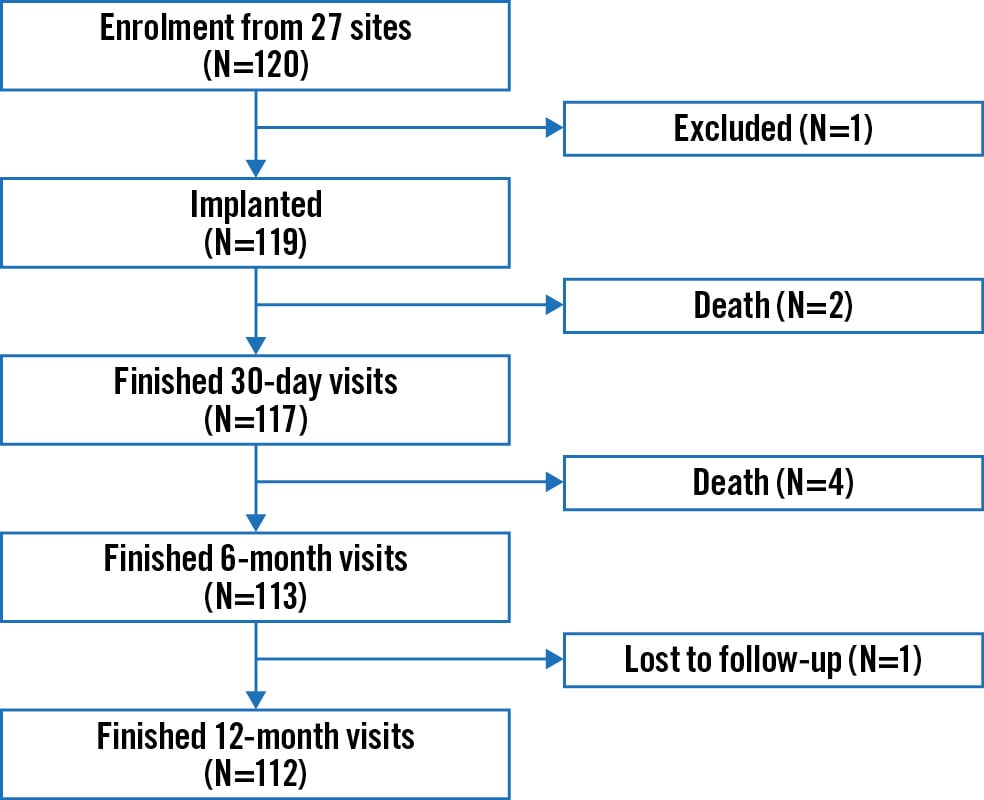
Figure 1. Flowchart for safety and effectiveness study of the DragonFly System for degenerative mitral regurgitation. Illustration of patient enrolment and follow-up, with visit windows of 30±7 days, 6 months±30 days, and 12 months±30 days.
Table 1. Baseline characteristics.
| Baseline characteristic | Degenerative MR (N=120) |
|---|---|
| Demographics | |
| Age, years | 74.9±5.7 (120) |
| Female | 59 (49.2) |
| BMI, kg/m2 | 22.6±3.2 (120) |
| BSA, m2 | 1.6±0.2 (120) |
| NYHA Class III/IV | 79 (65.9) |
| KCCQ score | 44.9±18.4 (120) |
| STS* score for mitral valve replacement | 6.9±2.8 (120) |
| Medical history/comorbidity | |
| Coronary artery disease | 47 (39.2) |
| MI | 5 (4.2) |
| CABG | 0 (0) |
| PCI | 19 (15.8) |
| Other cardiovascular diseases | 90 (75.0) |
| Other non-cardiovascular diseases | 103 (85.8) |
| Cardiovascular intervention/surgery | 22 (18.3) |
| Prior cardiac surgery | 0 (0) |
| Transcatheter aortic valve intervention | 1 (0.8) |
| Pacemaker implantation | 2 (1.7) |
| ICD | 0 (0) |
| Severe symptomatic carotid stenosis | 0 (0) |
| Acute peptic ulcer or gastrointestinal bleeding | 4 (3.3) |
| COPD | 13 (10.8) |
| Diabetes | 26 (21.7) |
| Hypertension | 85 (70.8) |
| CVA | 16 (13.3) |
| Active infection** | 7 (5.8) |
| Allergy*** | 13 (10.8) |
| Modified Rankin score | 0.9±1.1 (17) |
| Values are mean±SD (N) or N (%). For continuous variables, p-values were based on the Kruskal-Wallis test; for categorical variables, the p-values were based on Fisher’s exact test. * Per protocol, a surgical valve replacement STS score of ≥8, surgical valve repair STS score of ≥6 or the presence of other surgical high-risk factors such as the presence of ≥2 moderate to severe indicators of frailty or the presence of possible surgical operative impairment or the presence of ≥2 major organ dysfunctions that will not improve in the postoperative period or other surgical high-risk factors judged by the cardiology team were recommended as inclusion criteria. As most of the published articles report the surgical valve replacement STS score, here we also show it to be comparable. ** Active infection means infection requiring current antibiotic therapy. According to the inclusion criteria, patients may be enrolled at least 14 days after discontinuation of antibiotics. In 6 of the 7 patients there was a history of recent pulmonary infection, and in the remaining patient there was a recent history of an upper respiratory tract infection.*** Nine of the 13 patients had a history of being allergic to penicillins, 2 to sulfonamides, one to tetracycline, and one to perindopril/indapamide. BMI: body mass index; BSA: body surface area; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident; ICD: implantable cardioverter-defibrillators; KCCQ: Kansas City Cardiomyopathy Questionnaire; MI: myocardial infarction; MR: mitral regurgitation; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SD: standard deviation; STS: Society of Thoracic Surgeons | |
Table 2. Echocardiographic measures at baseline.
| Echocardiographic measure | Degenerative MR (N=120) |
|---|---|
| Degenerative mitral regurgitation aetiology | 120 (100) |
| LVEF, % | 60.8±7.8 (120) |
| MR grade | |
| 0 | 0 (0) |
| 1+ | 0 (0) |
| 2+ | 0 (0) |
| 3+ | 32 (26.7) |
| 4+ | 88 (73.3) |
| EROA, cm2 | 0.5±0.2 (107) |
| RV, ml | 76.9±24.8 (105) |
| TAPSE, mm | 19.9±3.1 (81) |
| MVOA, cm2 | 5.8±1.2 (120) |
| TMPG, mmHg | 2.5±1.3 (119) |
| Length of anterior leaflet, cm | 2.3±0.4 (120) |
| Length of posterior leaflet, cm | 1.4±0.3 (120) |
| Leading MR mechanism | |
| Prolapse | 91 (75.8) |
| Flail | 26 (21.7) |
| Prolapse plus flail | 3 (2.5) |
| Bileaflet prolapse | 2 (1.7) |
| Prolapse width, mm | 14.1±3.8 (120) |
| Prolapse/flail gap, mm | 3.5±1.9 (120) |
| Prolapse locationa, N (%) | |
| Posterior leaflet | |
| P2 | 67 (55.8) |
| Non-P2 | 34 (28.3) |
| Anterior leaflet | |
| A2 | 16 (13.3) |
| Non-A2 | 5 (4.2) |
| Bileaflet | |
| A2P2 | 1 (0.8) |
| A3P3 | 1 (0.8) |
| PASP, mmHg | 43.5±12.8 (98) |
| LAV, ml | 114.1±45.9 (117) |
| LAVi, ml/m2 | 70.2±29.1 (117) |
| LVESV, ml | 48.2±20.1 (120) |
| LVEDV, ml | 121.3±39.0 (120) |
| LVESD, cm | 3.2±0.7 (120) |
| LVEDD, cm | 5.1±0.6 (120) |
| TR | |
| No regurgitation | 18 (15.0) |
| Mild | 63 (52.5) |
| Moderate | 37 (30.8) |
| Severe | 2 (1.7) |
| Values are mean±SD (N) or N (%). For continuous variables, p-values were based on the Kruskal-Wallis test; for categorical variables, the p-values were based on Fisher’s exact test. MR grade was evaluated on transoesophageal echocardiography by the echocardiography core lab. aThere were two patients with prolapses involving both anterior and posterior leaflets: 1 patient’s prolapse involved A3/P3/A2 (1.8%) and the other patient’s involved A2/P2 (1.8%). EROA: effective regurgitation orifice area; LAV: left atrial volume; LAVi: left atrial volume index; LVEDD: left ventricular end-diastolic diameter; LVEDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVESV: left ventricular end-systolic volume; MR: mitral regurgitation; MVOA: mitral valve orifice area; PASP: pulmonary artery systolic pressure; RV: regurgitation volume; TAPSE: tricuspid annular plane systolic excursion; TMPG: transmitral mean pressure gradient; TR: tricuspid regurgitation | |
PROCEDURAL OUTCOMES
The success rate of the DragonFly device implantation was 99.2% (119/120); one patient’s treatment was not successful because of inadequate MR reduction, leading to device removal. The median device implantation time was 90.0 (58.5-117.0) min, the median procedural time was 109.0 (75.5-143.5) min, and the median fluoroscopy time was 29.5 (19.5-41.9) min. One DragonFly device was successfully implanted in 52.5% (63/120) of patients and 2 devices in 42.5% (51/120). The additional procedural measures are shown in Table 3. A learning curve analysis revealed a trend of reduced procedural time with experience from 1 to >3 DragonFly procedures performed (median 82.0 min and 74.5 min) and from 2 to >3 procedures performed. A similar reduction trend was seen in device time (median 68.5 min and 60.5 min) with procedures involving implantation of only one device (Supplementary Figure 1, Supplementary Figure 2).
Table 3. Procedural measures.
| Procedural measure | Degenerative MR (N=120) |
|---|---|
| Successful device implantationa | 119 (99.2) |
| Device implantation time, minb | 90.0 [58.5-117.0] (120) |
| Procedural time, minc | 109.0 [75.5-143.5] (120) |
| Fluoroscopy time, min | 29.5 [19.5-41.9] (120) |
| Number of devices implanted | |
| 0d | 1 (0.8) |
| 1 | 63 (52.5) |
| 2 | 51 (42.5) |
| 3 | 4 (3.3) |
| 4 | 1 (0.8) |
| Number of devices implanted | 1.5±0.6 (120) |
| Size of device | 181 (100) |
| SN0409 | 16 (8.8) |
| XN0412 | 25 (13.8) |
| SW0609 | 31 (17.1) |
| XW0612 | 109 (60.3) |
| Implantation location | 181 (100) |
| A1P1 | 17 (9.4) |
| A2P2 | 129 (71.3) |
| A3P3 | 35 (19.3) |
| Values are presented as N (%), median [IQR] (N), or mean±SD (N). For continuous variables, p-values were based on the Kruskal-Wallis test; for categorical variables, p-values were based on Fisher’s exact test. a Successful implantation: successful delivery and deployment of one or more devices, confirmed by echocardiography to demonstrate leaflet coaptation, and retrieval of the delivery catheter. b Device implantation duration: from when the guide sheath reaches the left atrium to when the device delivery system returns to the guide sheath. c Procedural duration: from transseptal start to guide sheath removal from the left atrium. d Device needed to be withdrawn in one patient because of anatomical reasons. IQR: interquartile range; MR: mitral regurgitation; SD: standard deviation | |
PRIMARY EFFICACY ENDPOINT
Kaplan-Meier survival analysis was used to evaluate the full analysis set and demonstrated a clinical success rate of 87.5% at 12 months after the procedure (95% CI: 80.1-92.3) (Central illustration B), surpassing the target value of 60% (p<0.001) prespecified in the statistical analysis plan (Supplementary Appendix 2). All patients included in the baseline data had an MR grade ≥3+, as adjudicated by the independent echocardiographic core laboratory. At discharge, 30-day, 6-month, and 12-month follow-ups, the proportion of patients with MR grade ≤2+ was 100%, 90.4%, 93.0%, and 92.0% in the unpaired analysis, respectively. Compared with baseline, these improvements were statistically significant (p<0.001). The MR grade was ≤1+ with rates of 85.6%, 64.3%, 62.6%, and 69.7%, respectively, in the unpaired analysis (Central illustration C). Patients with MR ≤1+ at 30 days post-procedure maintained a good reduction at 6- and 12-month follow-ups (Supplementary Figure 3).
SAFETY ENDPOINTS
The Kaplan-Meier estimates for freedom from all-cause mortality, reintervention for mitral valve dysfunction, HFH, and a composite of the above events were 95.0%, 95.0%, 96.6%, and 92.5%, respectively (Figure 2).
All mortality and aetiologies were adjudicated by the CEC. The composite MAE rate at 1 year was 9.2% (11/120), with five (4.2%) cardiovascular mortalities, three (2.6%) strokes, three (2.6%) renal failures, and three (2.6%) cardiovascular surgeries/procedures related to device or procedural complications, with two patients receiving pericardiocentesis and repair of arteriovenous fistula at the puncture site (Supplementary Table 5). Six patients died, resulting in an all-cause mortality rate of 5.0%. Two patients died during the 30-day follow-up period, and four died between 30 days and 6 months. One patient had multisegment degenerative disease (Barlow’s valve) and received 3 devices, but with inadequate MR reduction, prompting elective surgical mitral valve replacement, and then died 28 days post-procedure (26 days after the surgery). One patient died of new onset infection and septic shock at 30 days. Three patients died of unknown causes, and one died of severe pneumonia at 6 months. All of the preceding complications occurred during the COVID-19 pandemic.
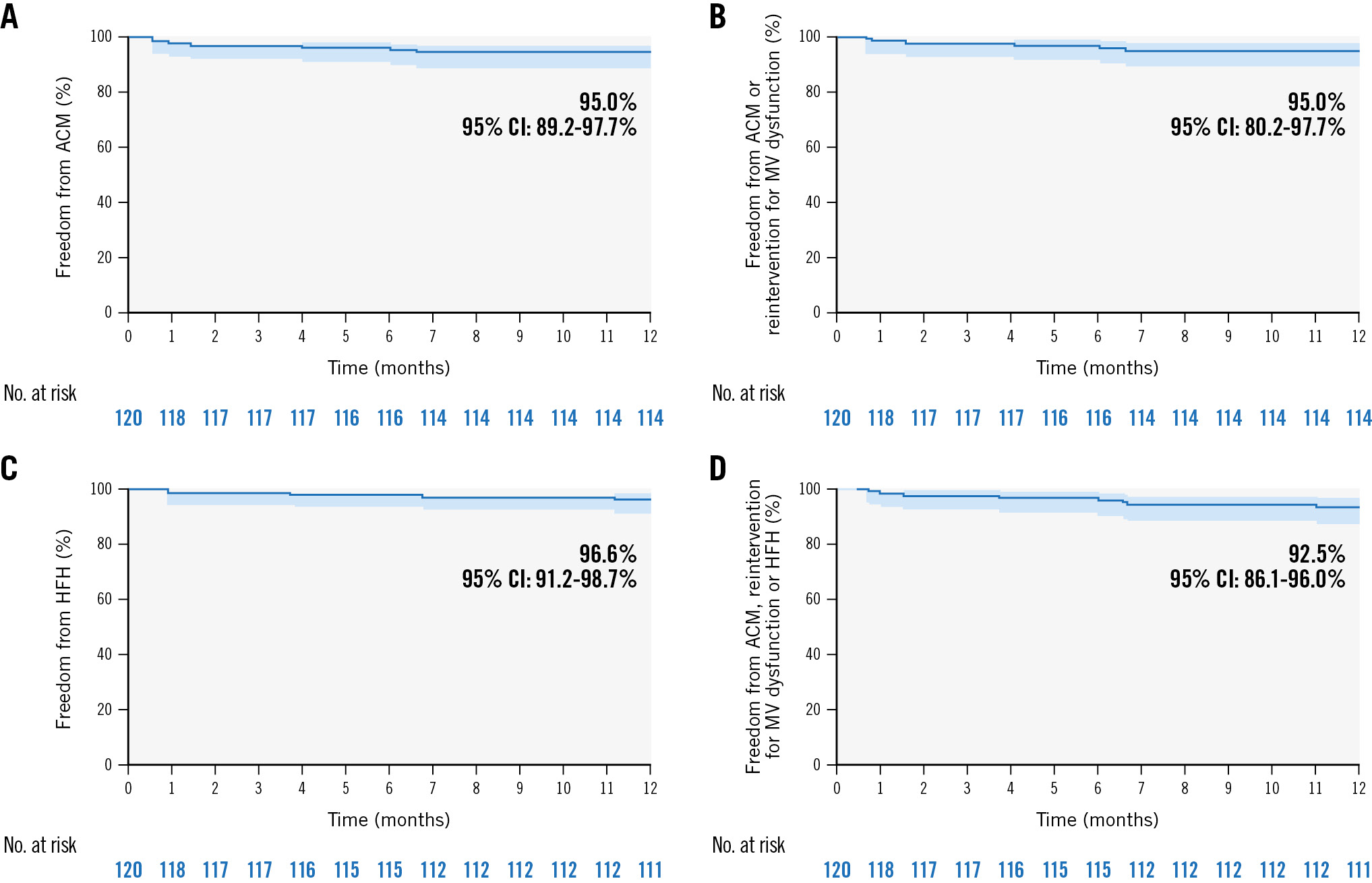
Figure 2. Kaplan-Meier analyses for estimating the freedom from all-cause mortality, reintervention for MV dysfunction and HFH at 1 year. Kaplan-Meier estimates for freedom from (A) all-cause mortality, (B) all-cause mortality or reintervention for MV dysfunction, (C) HFH, and (D) a composite of events rates at 1 year. Error bars represent 95% CI. ACM: all-cause mortality; CI: confidence interval; HFH: heart failure hospitalisation; MV: mitral valve
FUNCTIONAL EVALUATION
There were significant improvements in functional capacity and quality-of-life (QOL) outcomes (all p<0.05) compared to baseline in the paired analysis. At 12 months, the site-assessed NYHA Class showed significant improvement, with the percentage of patients categorised as Class I/II increasing from 32.4% at baseline to 93.6% at 12 months (p<0.001). Assessment using the KCCQ showed a mean improvement in self-assessed HF symptoms of 31.1±18.2 from baseline to 12 months (p<0.001) (Figure 3). The unpaired analysis showed similar results (Supplementary Figure 4).
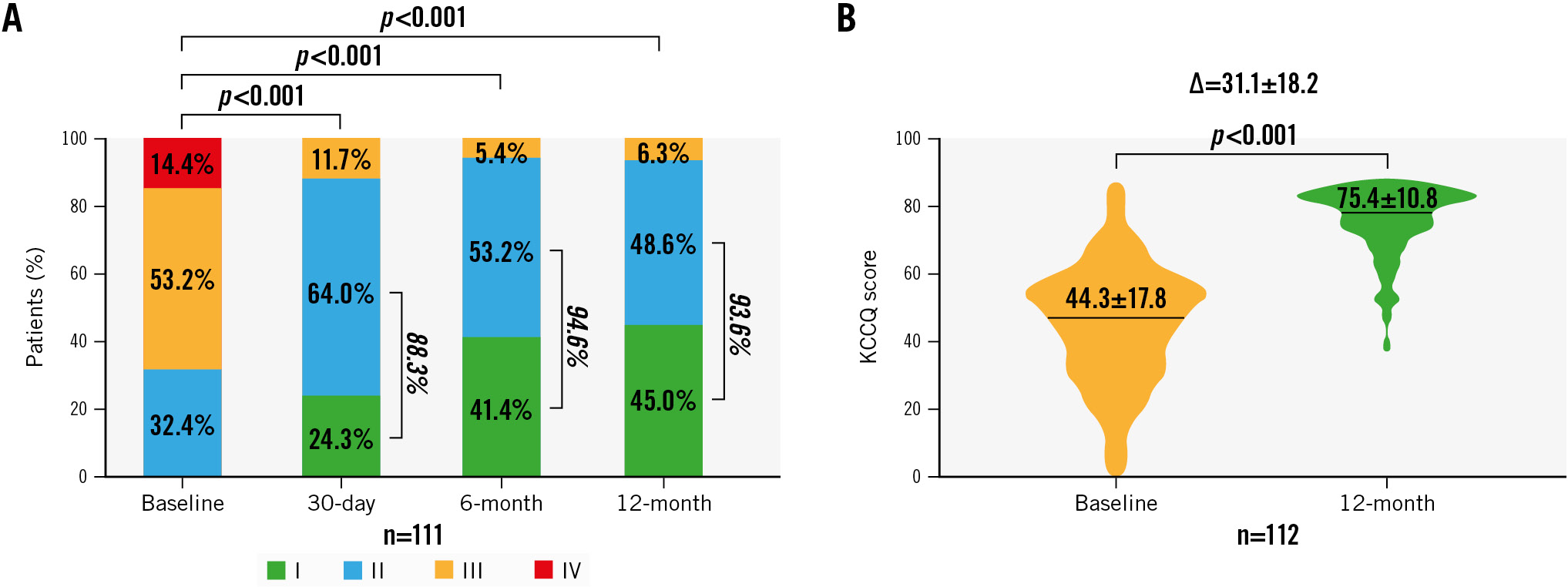
Figure 3. Paired analysis for NYHA Functional Class and KCCQ outcomes at baseline and follow-up. The graph shows the paired analysis of (A) New York Heart Association (NYHA) Functional Classification and (B) Kansas City Cardiomyopathy Questionnaire (KCCQ) scores. The p-values for group comparisons were calculated using the Student’s t-test for continuous variables and the Wilcoxon signed-rank test for categorical variables.
ADDITIONAL OUTCOME MEASURES
There was a significant reduction in postoperative left atrial pressure compared to the screening phase from 16.1±7.0 mmHg to 11.3±6.0 mmHg (p<0.001) (Supplementary Figure 5). Compared to baseline, the transmitral mean pressure gradient (TMPG) increased from a mean of 2.4±1.3 mmHg to 3.0±1.3 mmHg (p<0.001). However, it remained stable within a low range during follow-up (at 12 months: 3.2 mmHg; p=0.06) (Supplementary Figure 6). A paired analysis showed a decrease in left ventricular end-diastolic volume (LVEDV) by 19.4±32.9 ml and a decrease in left ventricular end-systolic volume by 9.8±18.1 ml (Supplementary Figure 7). Other echocardiographic data at follow-up are described in Supplementary Table 6.
Discussion
DMR is a prevalent disease globally1718. If left untreated, severe DMR will lead to a poor prognosis3. However, undertreatment is common because of high operative risk, under-referral and aversion to surgery78. In the USA and Europe, mitral TEER has emerged as a safe and effective treatment option for patients with DMR who are at a prohibitive surgical risk5. MitraClip and PASCAL are two TEER devices that have received U.S. Food and Drug Administration and European conformity (CE mark) approvals11. The DragonFly transcatheter valve repair system adds to the TEER family of devices with specific innovations for treating DMR. The DRAGONFLY-DMR pivotal trial is a prospective, multicentre, single-arm, performance goal study conducted to evaluate the safety and efficacy of a new TEER device in patients with DMR who are not eligible for surgery. This study has several significant findings. Notably, the study achieved its prespecified primary efficacy endpoint, clinical success rate, which measured freedom from all-cause mortality, mitral valve reintervention, and MR >2+ at the 1-year follow-up. The clinical success rate observed in the current study at 1 year (87.5%, 95% CI: 80.1-92.3) surpassed a more contemporary performance benchmark (60.0%) derived from a meta-analysis of mitral TEER studies on DMR conducted between 2011 and 2021 (Supplementary Appendix 2)91019202122. Additionally, the study demonstrated a reassuring safety profile for the DragonFly system. The trial also observed a high degree of acute reduction in MR to 1+, which was sustained from 1 month to 1 year. Furthermore, the postprocedural mitral inflow gradients were low and remained stable, and the study showed a remarkable improvement in QOL during the follow-up period.
The TEER procedure is a relatively new technique in China compared to the USA and Europe, where this technique has a history of more than 15 years and has been utilised in more than 150,000 cases. Even though DragonFly TEER was performed by operators who were inexperienced with the TEER procedure, the incidence of MAE shown in this study was notably low from 30 days to 1 year post-procedure (Supplementary Table 5), showing a favourable safety profile for the DragonFly over the early experience of MitraClip22. All-cause mortality, cardiovascular mortality, and HFH rates at 1 year were 5.0% (95% CI: 2.3-10.8), 4.2% (95% CI: 1.8-9.8) and 3.4% (95% CI: 1.3-8.8), respectively. Single-leaflet device attachment occurred in only 1 patient (0.8%) at the 1-month follow-up. These findings were comparable to those of the CLASP IID study11 and the STS/American College of Cardiology Transcatheter Valve Therapy Registry conducted using two other major mitral TEER devices23. Despite the vertical design of the grippers’ leaflet retention elements, which are similar to those of the MitraClip but different from the horizontal design of PASCAL, no leaflet injury was identified as occurring during the study. Furthermore, there were no incidents of chordal entrapment with the implant, which may be attributed to the narrow and slim design of the arms across all four DragonFly sizes, and the capability of both clip arms to revert to an obtuse angle (270°), which facilitates clip repositioning underneath the leaflets and manoeuvring among dense chordae close to the commissures.
In the current trial, 90.0% of patients achieved MR reduction to grade 2+ or less at 1 month; notably, 65.5% of patients achieved MR reduction to 1+. Importantly, residual MR ≤1+ has been associated with better clinical outcomes compared with residual MR ≤2+2425, and 91.2% of those with a reduction of MR to ≤1+ at 1 month maintained the effect 1 year later (Supplementary Figure 3). These findings were consistent with the findings from CLASP IID and underscore the importance of resolving MR as much as possible through mitral TEER. With a decrease of 19.4±32.9 ml in LVEDV compared to baseline at 12 months, DragonFly showed its effect on left ventricular reverse remodelling, which was comparable or even favourable to the historical data from MitraClip/PASCAL devices in the current study22. In addition, even with the complexity of prolapse anatomies at baseline (Table 1), the mean number of devices implanted in this trial to achieve the aforementioned MR reduction was low and comparable to the results of more recent studies with the MitraClip and PASCAL devices1122. Moreover, the procedure and device times were surprisingly shorter than the time taken by physicians in the USA and Europe to implant MitraClip at a similarly early stage of mitral TEER22. Taking into account that the trial was conducted in China, where the TEER procedure is relatively new to the physicians, as well as restrictions due to the coronavirus disease pandemic, proctoring for these cases with TEER experts overseas could only be done through remote online teaching. Most sites had no prior mitral TEER experience. A reduced procedural time was observed with greater operator experience (Supplementary Figure 2), demonstrating the intuitiveness and procedural efficiency of the DragonFly system. The Transcatheter Valve Therapy Registry reported a comparable pattern of learning curves, revealing that there is a correlation between experience and a reduction in procedural time when using TEER devices in real-world scenarios26. Further improvement in procedural time can be anticipated if operators gain greater experience with the DragonFly system.
Furthermore, the acute increase in TMPG levels after the DragonFly TEER was insignificant. At 1 year, the entire cohort of patients maintained a satisfactorily low TMPG, with a mean inflow gradient of 3.2±1.4 mmHg (Supplementary Figure 6). The acute decrease in left atrial pressure was significant and likely contributed to significant symptomatic improvement (Supplementary Figure 5). Along with the durable reduction in MR and left ventricular reverse remodelling over time, the impact of mitral TEER with DragonFly on patients with DMR in this study translated to the alleviation of HF symptoms, improvement in patients’ NYHA Functional Class and QOL, and a significant increase in KCCQ score compared to baseline (Δ=31.1±18.2).
The differentiating features of the DragonFly device compared to other TEER devices are the central compressible filler, the mechanically locked arms of the device, and the indexed articulation of the delivery system. Unlike the MitraClip device but similarly to the PASCAL device, the central compressible filler acts to block the regurgitant orifice without pulling the mitral leaflets so tightly together, as they are instead brought towards the filler. However, unlike the PASCAL device’s central non-compressible spacer, the DragonFly’s filler is compliant, allowing it to be compressed by the device arms, which in turn distends medially and laterally, further blocking the regurgitant orifice. Similar to the PASCAL Ace (Edwards Lifesciences) device, this allows operators to pull redundant leaflet tissue closer together when necessary. This is a unique feature of the DragonFly implant that may have contributed to the significant and sustained MR reduction while maintaining the inflow gradient at a low level over time. Also, this potentially allows operators to use the DragonFly device to address a wider regurgitation orifice using fewer devices, leaving a larger mitral valve orifice area post-procedure and a lower residual inflow gradient. In addition, operators have the option to decrease the tension on both leaflets after device implantation by mechanically locking the arms of DragonFly at a range of angles, and implantation is secured by firmly sandwiching the leaflets between the arms and the central filler to maximise leaflet coaptation. The robust mechanical locking force of the device delivers clamping force to the leaflets, even in complicated cases of fibrotic leaflets or those with scattered leaflet calcification in the grasping area. Other features, such as the same articulating mechanical delivery system shared by both the mitral and tricuspid sides and gauge windows of the DragonFly system, are designed to provide a shortened learning curve for operators, promoting confidence in making stable, precise, and replicable movements at each step during the procedure. The availability of different sized TEER devices expands the treatment options for patients with DMR and accommodates various anatomical and mitral aspects. The unique design characteristics of the DragonFly device may add new possibilities to the TEER armamentarium and lead to operator preference for complex mitral anatomies. For instance, the suitability of DragonFly in treating DMR in patients with a smaller baseline MVOA, especially those with prior annuloplasty or a higher baseline mitral inflow gradient, is sure to arouse clinical interest and warrants further studies.
Table 1. Baseline characteristics.
| Baseline characteristic | Degenerative MR (N=120) |
|---|---|
| Demographics | |
| Age, years | 74.9±5.7 (120) |
| Female | 59 (49.2) |
| BMI, kg/m2 | 22.6±3.2 (120) |
| BSA, m2 | 1.6±0.2 (120) |
| NYHA Class III/IV | 79 (65.9) |
| KCCQ score | 44.9±18.4 (120) |
| STS* score for mitral valve replacement | 6.9±2.8 (120) |
| Medical history/comorbidity | |
| Coronary artery disease | 47 (39.2) |
| MI | 5 (4.2) |
| CABG | 0 (0) |
| PCI | 19 (15.8) |
| Other cardiovascular diseases | 90 (75.0) |
| Other non-cardiovascular diseases | 103 (85.8) |
| Cardiovascular intervention/surgery | 22 (18.3) |
| Prior cardiac surgery | 0 (0) |
| Transcatheter aortic valve intervention | 1 (0.8) |
| Pacemaker implantation | 2 (1.7) |
| ICD | 0 (0) |
| Severe symptomatic carotid stenosis | 0 (0) |
| Acute peptic ulcer or gastrointestinal bleeding | 4 (3.3) |
| COPD | 13 (10.8) |
| Diabetes | 26 (21.7) |
| Hypertension | 85 (70.8) |
| CVA | 16 (13.3) |
| Active infection** | 7 (5.8) |
| Allergy*** | 13 (10.8) |
| Modified Rankin score | 0.9±1.1 (17) |
| Values are mean±SD (N) or N (%). For continuous variables, p-values were based on the Kruskal-Wallis test; for categorical variables, the p-values were based on Fisher’s exact test. * Per protocol, a surgical valve replacement STS score of ≥8, surgical valve repair STS score of ≥6 or the presence of other surgical high-risk factors such as the presence of ≥2 moderate to severe indicators of frailty or the presence of possible surgical operative impairment or the presence of ≥2 major organ dysfunctions that will not improve in the postoperative period or other surgical high-risk factors judged by the cardiology team were recommended as inclusion criteria. As most of the published articles report the surgical valve replacement STS score, here we also show it to be comparable. ** Active infection means infection requiring current antibiotic therapy. According to the inclusion criteria, patients may be enrolled at least 14 days after discontinuation of antibiotics. In 6 of the 7 patients there was a history of recent pulmonary infection, and in the remaining patient there was a recent history of an upper respiratory tract infection.*** Nine of the 13 patients had a history of being allergic to penicillins, 2 to sulfonamides, one to tetracycline, and one to perindopril/indapamide. BMI: body mass index; BSA: body surface area; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CVA: cerebrovascular accident; ICD: implantable cardioverter-defibrillators; KCCQ: Kansas City Cardiomyopathy Questionnaire; MI: myocardial infarction; MR: mitral regurgitation; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SD: standard deviation; STS: Society of Thoracic Surgeons | |
Limitations
This study has some limitations. Firstly, the study design was a single-arm performance goal trial, as positive control devices such as the MitraClip and experience with the TEER technique were inaccessible in China during the trial design and execution. This may introduce bias and limit our ability to draw conclusions. However, to mitigate potential bias, the study employed several designs, such as clear inclusion/exclusion criteria, a centralised screening committee for patient enrolment, objective outcome measures as primary endpoints, a standardised protocol across participating sites, an independent image core laboratory, and an independent clinical event committee to ensure objectivity and data accuracy. Secondly, the follow-up period for the primary effectiveness endpoint was relatively short, which may have hindered the assessment of the long-term benefits and safety of the treatment. Finally, the study was conducted during the coronavirus disease pandemic, which may have affected patient follow-up and data collection. Nevertheless, the study findings are encouraging and promising. Future research with larger sample sizes and longer follow-up periods is necessary to validate the results and assess the long-term benefits and safety of this treatment.
Conclusions
In summary, the DragonFly transcatheter valve repair system has been investigated in this current DRAGONFLY-DMR study, which represents the first pivotal trial to evaluate the safety and efficacy of this system in patients with significant symptomatic DMR (3+ and 4+, as adjudicated by the independent echocardiographic core laboratory) who were at high risk for mitral valve surgery. The trial’s prespecified primary efficacy endpoint was successfully achieved, and the results demonstrated that treatment with the DragonFly system had a low occurrence of adverse events and delivered sustained MR reduction with low mitral inflow gradient and left ventricular reverse remodelling over time. The therapy successfully demonstrated its safety and efficacy in treating degenerative mitral regurgitation and in improving patients’ quality of life, indicating its potential as a beneficial therapy for such patients.
Impact on daily practice
The DRAGONFLY-DMR trial provides important evidence for the safety and efficacy of the DragonFly transcatheter valve repair system in treating patients with symptomatic chronic DMR 3-4+ at high surgical risk. The unique design characteristics of DragonFly may expand new possibilities in the TEER armamentarium and lead to operator preferences in complex mitral anatomies. The suitability of DragonFly in treating DMR patients with a smaller baseline MVOA, especially those with prior annuloplasty or a higher baseline TMPG, may be of clinical interest and warrants further studies.
Acknowledgements
The authors thank all patients and sites that participated in the trial and Mengmeng Gong, MS, Yi Duan, MS, Xiaoyue Tang, MS, and Guoliang Wu, MS, for their assistance with manuscript preparation.
Conflict of interest statement
K. Ma receives a salary from Valgen as Chief Medical Officer. S. Lim receives personal consulting fees from LagunaTech, Philips, Valgen, and Venus; and his institution receives research grants on his behalf from Abbott, Boston Scientific, Corvia, Edwards Lifesciences, Medtronic, V-Wave, and W.L.Gore & Associates. All other authors report their institutions receiving institutional research grants from Valgen for the conduct of the study.
Supplementary data
To read the full content of this article, please download the PDF.

