Abstract
Aims: Clinical trials showed contradictory results in functional recovery after intracoronary infusion of autologous mononuclear (bone marrow) cells in patients with acute myocardial infarction. A recent study suggests that this might be related to the isolation protocol used. In The Netherlands, a comparable randomised multicentre trial (HEBE) was designed. To validate the isolation method of bone marrow and peripheral blood-derived mononuclear cells, we compared our processing protocol with methods comparable to the ASTAMI (no beneficial effect) and the REPAIR-AMI study (beneficial effect).
Methods and results: The effect of several factors (density gradient, washing buffer and centrifugation speed) has been studied on recovery and function (migration and clonogenic capacity) of mononuclear cells. Significantly lower cell recoveries were found at a centrifugation speed of 250 g, compared to 600 or 800 g, respectively. Furthermore, washing buffer without supplemented human serum albumin and heparin resulted in significantly lower cell recovery and functional impairment as measured by clonogenic capacity.
Conclusions: The results of our study justify the cell-processing protocol as applied in the HEBE trial (600 g, human serum albumin supplemented washing buffer). This protocol results in viable and functional cells of which the quantity and quality is at least comparable to a successful study like the REPAIR-AMI.
Introduction
A number of clinical studies have been documented in which (subsets of) bone-marrow derived cells (BMC) are intracoronary administrated after acute myocardial infarction followed by percutaneous coronary intervention (and which are reviewed in reference number 1). Only part of the published studies are randomised, controlled trials2-6 and although the available evidence given in a recently published meta-analysis suggests that BMC transplantation is associated with modest improvement in myocardial function, results from some of these studies are contradictory7-9. The REPAIR-AMI trial, the largest study so far in which 204 patients were randomised, showed that the absolute improvement of left ventricular ejection fraction was enhanced among patients treated with BMC compared to placebo4,10. In the ASTAMI trial (n=101), no benefit of intracoronary BMC infusion was observed11,12. In both these evaluated randomised, multicentre studies, the mononuclear cells were isolated with a gradient separation protocol and (sometimes after overnight storage) re-infused intracoronary. However, there were differences between the cell isolation protocols used, and Seeger et al showed that these had a major impact on recovery, viability and functionality of the cells13.
In The Netherlands, the HEBE trial was designed. The aim of the HEBE trial is to include 200 patients, divided over three treatment arms. Patients are randomised to treatment with: (1) intracoronary infusion of autologous BMC, (2) intracoronary infusion of autologous peripheral blood mononuclear cells (PBMC), or (3) standard therapy14.
The mononuclear cells are isolated from the collected bone marrow or venous blood, by density gradient centrifugation. Lymphoprep is used as density gradient (separation medium) and saline supplemented with human serum albumin (HSA, 4% v/v) and heparin (0.4% v/v) as washing medium at a centrifuge speed of 600 g. After processing, the cells are re-suspended in saline with 4% HSA supplemented with heparin (0.4% v/v) and infused intracoronary at the day of collection14.
To validate the isolation method of bone marrow as well as the peripheral blood-derived mononuclear cells of the HEBE trial, we compared our processing protocol with the methods used in previous trials. Several quantitative and qualitative in vitro parameters were tested: cell recovery, viability, haematopoietic stem/progenitor cell (HSC) count and chemokine receptor (CXCR4) expression were determined by flow cytometric analyses. In addition, semi-solid Colony Forming Unit-Granulocyte-Macrophage (CFU-GM) cultures and a migration assay were performed to test the functionality of the isolated (progenitor) cells.
Our study demonstrates that the cell-processing protocol applied in the HEBE trial results in a cell fraction of which the quantity and quality is at least comparable to a successful study like the REPAIR-AMI. We show that the choice of density gradient solution has no effect on cell recovery; however, the composition of the washing medium and centrifugation speed directly influences cell recovery and functional activity of the isolated cells.
Methods
Cell isolation protocols
Bone marrow (BM) was collected from the sternum of patients undergoing cardiac surgery at the Academic Medical Center (AMC). The Medical Ethical Review Board of the AMC approved the protocol for collecting bone marrow for research purposes. Peripheral blood (PB) was drawn from healthy volunteers. All patients and volunteers gave written informed consent.
First, cells were isolated from the heparinised material according to the scheme in Figure 1A.
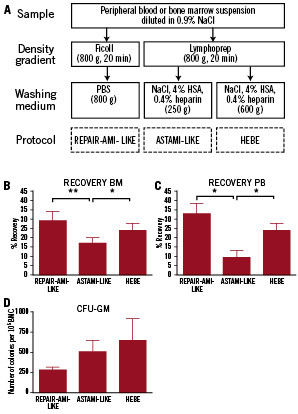
Figure 1. Recovery and clonogenic potential of BMC and PBMC after cell isolation via the REPAIR-AMI-like, ASTAMI-like and HEBE protocol. (A) Schematic drawing of the used isolation protocols. (B,C) The recovery of mononuclear cells following the ASTAMI-like protocol was lower than the recovery of the REPAIR-AMI-like and HEBE protocols. (B) Bone marrow (BM) (17.2±3.0% vs.29.0±5.1% vs. 24.0±3.7% respectively, n=6). (C) Peripheral blood (PB) (9.6±3.8% vs. 33.4±5.5% vs. 24.2±3.8%, n=3). (t-test, * p=< 0.05, ** p<0.01) (D) Clonogenic potential of BMC. The REPAIR-AMI-like method shows a decreased colony formation compared to the ASTAMI-like and HEBE protocol (283±36 vs. 513±137 vs. 652±268 colonies per 105 cells, n=3), although this does not reach significance.
Bone marrow aspirate or peripheral blood was diluted in 0.9% NaCl, and mononuclear cells were isolated using a) Ficoll, 20 min, 800 g (Pharmacia Biotech, Uppsala, Sweden), washed with phosphate buffered saline (PBS), centrifuge speed 800g (REPAIR-AMI-like) or b) Lymphoprep, 20 min, 800 g (AXIS-SHIELD PoC AS, Norway), washed with 0.9% NaCl, 4% HSA, 0.4% heparin (20, IU/ml), centrifugation speed 250g (ASTAMI-like protocol) or c) Lymphoprep and washed with 0.9% NaCl, 4%HSA, 0.4% heparin, centrifugation speed 600 g (HEBE protocol). Every bone marrow aspirate or peripheral blood sample was divided and processed in parallel according to all three protocols, resulting in three different cell fractions. Cell recovery and clonogenic potential of these samples were determined.
For the experiments described in Figure 2 and Table 1, mononuclear cells from bone marrow aspirate or peripheral blood were isolated by density gradient centrifugation (centrifuge speed 800 g) using a) Lymphoprep or b) Ficoll. Subsequently, mononuclear cells were divided into two equal volumes and washed once at 600g with either a) NaCl supplemented with 4% HSA with 0.4% heparin or b) PBS, resulting in four different cell fractions, which were separately used in the experiments.
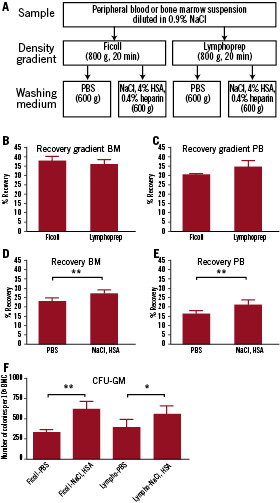
Figure 2. Recovery and clonogenic potential of BMC and PBMC after cell isolation with the conditions as drawn in (A). (B,C) Recovery after two different gradient separations; BMC (B) and PBMC (C) were isolated with both Ficoll and Lymphoprep. No difference in recovery was observed after the gradient separation (D, E). Recovery of mononuclear cells after washing with either PBS or NaCl following Ficoll or Lymphoprep gradient separation. NaCl supplemented with HSA with heparin gives a significant higher recovery than PBS. Bone marrow (BM): 23.0±2.9% vs. 27.2±3.0%, n=14 (D). Peripheral blood (PB): PBS vs. NaCl: 16.0±1.6% vs. 20.9±1.3%, n=8 (E). (F) Clonogenic potential of BMC after isolation via the four different protocols. NaCl induced a higher clonogenic outgrowth after Ficoll (337±72 vs. 627±117 colonies per 105 cells, n=7) and Lymphoprep (400±93 vs. 570±132 colonies per 105 cells, n=6). [t-test, *p<0.05, **p<0.01]
Cell recovery was determined by dividing the absolute number of cells after the subsequent isolation steps by the initial starting fraction.
Colony forming unit-granulocyte-macrophage assay
BMC were plated in duplicate in 35 mm tissue culture plates at concentrations of 1.0, 0.5 and 0.25*10 5 cells/ml, respectively, in MethoCult GF 4534 (StemCell Technologies, Vancouver, BC, Canada). Cultures were incubated for 12-14 days at 37 °C in a 5% CO2 humidified atmosphere and colony forming units, identified as colonies of over 40 translucent cells, were scored by microscopy.
Flow cytometry
Cell fractions were analysed by fluorescence-activated cell sorting (FACS) using the following directly conjugated antibodies against human cluster of differentiation (CD) 34 (HSC marker, PE-labelled, Pelicluster, Sanquin, Amsterdam, The Netherlands), CD45 (Leukocyte marker, FitC labelled, BD biosciences, San Jose, CA, USA) and CXCR4 (The receptor for the CXC chemokine stromal cell derived factor-1 (SDF-1) APC labelled, BD Biosciences). Flow count beads (Beckman Coulter, Fullerton, CA, USA) were added prior flow cytometry to calculate the absolute number of cells. In addition, viability was tested by 7-Amino-Actinomycin D (7AAD) staining for non-viable cells (BD Biosciences).
Migration
Migration assays were performed in Transwell plates (Costar, Cambridge, MA, USA) of 6.5 mm diameter filters with a pore size of 5 µm. The filters were coated overnight (O/N) with fibronectin (Sigma, St Louis, MO, USA). Approximately 200,000 mononuclear cells in 0.1 ml of assay medium (Iscove’s Modified Dulbecco’s Medium, 0.25% BSA) were seeded in the upper compartment and 0.6 ml of assay medium containing 100 ng/ml SDF-1 was added to the lower compartment. The Transwell plates were incubated O/N (BMC) or 4 h (PBMC) at 37 °C, 5% CO2. To analyse the migrated cells, FACS analysis was used. Cells prior and after migration in the lower well were stained for CD45, CD34 and CXCR4. In addition, to determine the percentage of migration, the absolute number of cells after migration was divided by the number of cells prior migration. From the BMC, the migration percentages of both leukocytes and haematopoietic stem cells (HSC) were determined. For PBMC, only the percentages of leukocyte migration could be determined, due to the small number of HSC in peripheral blood of healthy donors. Flow count beads were added to obtain absolute numbers of migrated cells.
Statistical analysis
Statistical differences were assessed using the paired t-test or Wilcoxon signed rank test. Significance was assumed at a p value < 0.05. Data are shown as mean±SEM.
Results
Comparison of the ASTAMI-like, REPAIR-AMI-like and HEBE isolation protocols.
Recovery
BMC and PBMC were isolated according to the schedule in Figure 1, resulting in three cell fractions. As shown in Figure 1, the recovery of mononuclear cells following the ASTAMI-like protocol was lower than the recovery of the REPAIR-AMI-like and HEBE protocols. This holds true for bone marrow (Figure 1B, 17.2±3.0%vs.29.0±5.1% (p<0.01) vs. 24.0±3.7% (p=0.05), respectively, n=6) and is even more pronounced for peripheral blood (Figure 1C, 9.6±3.8 vs. 33.4±5.5 [p<0.05] vs. 24.2±3.8 [p<0.05], respectively, n=3). Since the media used in the ASTAMI-like method and the HEBE method are equal, the reduced cell recovery from the first is most likely caused by the lower centrifugation speed used during washing.
Clonogenic potential
A reliable measurement for the functionality of HSC is the clonogenic potential of the cells in a CFU-GM assay. Figure 1D shows that the clonogenic potential of the BMC isolated by the REPAIR-AMI-like method is lower than the BMC isolated via the ASTAMI-like and HEBE protocol (283±36 vs. 513±137 vs. 652±268 colonies per 105 cells, n=3), although this did not reach statistical significance.
Since the ASTAMI-like isolation protocol and the HEBE isolation protocol differ from the REPAIR-AMI-like protocol in density gradient solution and the subsequent washing medium, it is likely that the difference in clonogenic outgrowth is caused by one of these variables.
Comparison of density gradient solution and washing media – Recovery
To validate our density gradient solution and washing media, cells from both bone marrow and peripheral blood were isolated following the scheme in Figure 2A.
First the effect of the different density gradient solutions was studied. Figure 2B and C show the recovery of cells from both bone marrow and peripheral blood after density separation of mononuclear cells with either Ficoll or Lymphoprep. No significant difference in cell recovery was observed between separation by Ficoll or Lymphoprep (Figure 2B, C).
Secondly, we determined the total cell recovery after density separation and a subsequent washing step. Since there was no difference in cell recovery between different density gradients, cell suspensions obtained after both Ficoll and Lymphoprep were used to determine the total cell recovery after washing. As shown in Figure 2D, a significant lower cell recovery was observed after the BMC were isolated and washed with PBS compared to NaCl with HSA and heparin (PBS 23.0±2.9% vs. NaCl 27.2±3.0%, p<0.001, n=14, Figure 2D). For peripheral blood, a comparable significant difference was observed (PBS vs. NaCl: 16.0±1.6% vs. 20.9±1.3%, p<0.001, n=8, Figure 2E).
Colony forming unit-granulocyte-macrophage assay
To determine clonogenic capacity after density separation and washing, cells from the four conditions were plated in semi-solid medium. When the washing buffers were compared, the BMC showed a significantly lower colony formation after washing with PBS, this holds true for both Ficoll (337±72 vs. 627±117 colonies per 105 cells; p<0.01, n=7, fig 2F) and Lymphoprep (400±93 vs. 570±132 colonies per 105 cells, p<0.05, n=6, fig 2F) solution.
Haematopoietic (stem) cell viability, recovery and migration
Since the washing medium has a major effect on cell recovery and CFU-GM potential, we determined whether it had impact on several other parameters as well.
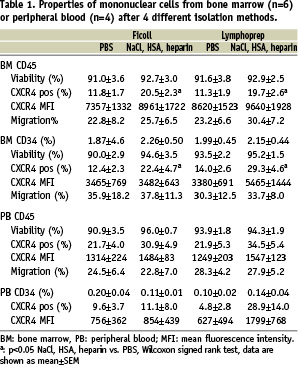
As can be observed in Table 1, the viability of the CD45+ leukocytes and the CD34+ cells did not differ between the separate conditions. Furthermore, we analysed the percentage of CXCR4 positive cells in the mononuclear cell fractions and CXCR4 expression on the leukocytes and CD34+ cells. The percentages of CXCR4 positive leukocytes and CD34+ cells were significantly higher in BMC washed with NaCl supplemented with HSA and heparin. For peripheral PBMC the same trend is observed, although this did not reach significance. No significant differences were observed in the mean fluorescence intensity of the CXCR4 expression on CD34+ cells and leukocytes from both bone marrow and peripheral blood.
To test whether the different isolation methods were of influence on functional capacity of cells, migration experiments were performed. All conditions showed equal migration of the mononuclear cells from both bone marrow and blood, indicating that the diminished CXCR4 expression is not reflected by a lower migratory capacity of the cells.
Discussion
The present study shows that the cell processing protocol as applied in the HEBE trial (600 g, and HSA/heparin supplemented washing buffer) results in a viable and functional cell fraction of which the quantity and quality is at least comparable to a successful study like the REPAIR-AMI. Moreover we show that the composition of the washing medium (and not the density gradient solution) affects the cell recovery and colony forming capacity of these cells.
In the first part of the study, we compared our cell-processing protocol (Lymphoprep and a washing step at centrifugation speed 600 g with NaCl supplemented with HSA with heparin), to conditions of the REPAIR-AMI trial (beneficial effect, Ficoll, PBS, 800 g) and ASTAMI trial (no beneficial effect, Lymphoprep, NaCl with HSA, 250 g). To avoid too many variables the tested protocols were not completely identical to the described study-protocols: for the “ASTAMI” conditions we used a higher (equal to HEBE protocol) HSA concentration (4% HSA instead of 1% heparinised plasma (~0.4% HSA)). Since overnight storage is not applied in the HEBE protocol, we did not include an overnight step in our experimental set-up.
The main observation is that the cell recovery of the ASTAMI-like method is significantly lower than that of the REPAIR-AMI-like and HEBE method. This is in concordance with the fact that number of infused cells described in the ASTAMI trial11 is approximately one third of the number of cells described in the pilot study of the HEBE trial, whereas the amount of bone marrow aspirated was similar (50 ml)15. The explanation for this lower cell recovery is most likely the lower centrifugation speed during washing, as was already suggested by Seeger et al13. However, when the quality of the isolated cells was investigated by a colony forming assay, the REPAIR-AMI-like method gave a lower (but not significant) outgrowth of colonies then the HEBE and ASTAMI-like method. This indicates a role for the density gradient solution or the washing media, since these are the differing factors that might affect the quality of the cells.
In the second part of our study we determined the influence of the washing media in a set-up without differing centrifuging speeds. No differences on cell recovery between Ficoll and Lymphoprep gradient separation were observed, which was to be expected, as both media contain identical concentration of ficoll and sodium diatrizoate16. In contrast, the NaCl washing medium that was supplemented with HSA and heparin turned out to be superior as compared to PBS for the recovery of mononuclear cells and their CFU-GM forming capacity, while the viability was unaffected. The unfavourable effect of PBS washing was also seen in the CXCR4 expression, which was decreased after washing with PBS, although this was not reflected in the migratory capacity of the cells. The negative effect of the PBS washing step can easily be explained by the fact that NaCl washing buffer was enriched with HSA and heparin, creating a more physiological environment for the cells. Thus for the HEBE trial we use a cell processing method that combines the best conditions from both the ASTAMI- and REPAIR-AMI protocols.
Differences in our experimental set-up (no use of bone marrow from healthy donors; no overnight storage) might explain our results that show an equal or even improved cell quality after using a method that resembles the ASTAMI protocol, which is in contrast to the findings of Seeger et al13. The results from Seeger were also based on in vivo experiments, which are not included in our experiments.
Whether the results of our study can predict whether the experimental arms in the HEBE study will be beneficial is unknown. The cell(s) responsible for a positive effect on cardiac repair are still unidentified. This study describes the functionality of mainly haematopoietic cells from the mononuclear cell fraction, while other candidate cells have been described that might influence cardiac repair e.g. endothelial progenitor cells17,18, myeloid cells19 and mesenchymal stem cells19-21 were not tested in our study. However, the cell suspensions that are infused in patients included in the HEBE trial will be analysed for the presence of several subpopulations, which then can be (retrospectively) correlated to the clinical outcome. Hopefully, these results will help us to identify specific mononuclear cell populations in peripheral blood and bone marrow that are most responsible for the observed clinical effects. Only then can cell therapy for cardiovascular therapy be optimised.
In conclusion, the isolation method used in the currently proceeding HEBE trial results in a mononuclear cell fraction consisting of viable and functional cells. The results of our current study justify the cell-processing protocol as applied in the HEBE trial (600g, and HSA/heparin supplemented washing buffer). Also, no overnight storage as used in the ASTAMI and REPAIR-AMI study is included in our protocol, which is even more beneficial for the quality of the cells. However, it is currently unclear whether the differences between the cell isolation procedures are responsible for the contrasting outcomes in the clinical trials published so far.
Acknowledgements
The authors would like to thank A.M. van der Laan for assistance in the collection of the bone marrow samples and the collaborators from the Laboratory for Stem Cell Transplantation Sanquin Research Amsterdam for their technical assistance.

