
Abstract
Aims: Avoiding side branch occlusion is challenging when treating bifurcation lesions. A newly designed stent system called the prewire channel stent (PWCS) with a side channel positioned between the metallic mesh material and the balloon is introduced. We aimed to compare the time taken to position the PWCS against that for a conventional stent.
Methods and results: The PWCS and a conventional stent were used in a pig model. The time taken from the starting point with the stent outside the body to reaching the bifurcation of the vessel ready for further procedures such as balloon dilatation through the stent mesh opening and double kissing balloon technique, etc., was compared in the conventional stent and PWCS groups. The time taken in the PWCS stent group included the time from sending the stent from outside the body to the desired position of the bifurcation of the vessels of the heart, releasing the stent and pulling back the balloon (SB time). The time taken in the conventional stent included the time from sending the stent from outside the body to the desired position of the bifurcation of the vessels of the heart, releasing the stent, pulling back the balloon (SB time), and wire exchange (WE time). The SB times for the PWCS and the conventional stent groups were not different (28.5±3.8 vs. 25.25±0.75 seconds, n=4). The PWCS group did not have “wire exchange,” and had no WE time, which was 28.5±5.7 seconds in the conventional stent group. The total time spent in the PWCS group was 28.5±3.8 seconds, which was shorter than the 53.75±6.2 seconds (n=4, p<0.05) in the conventional stent group.
Conclusions: The PWCS makes “wire exchange” in the side branch (SB) unnecessary and it can be as easily manipulated as a conventional stent.
Abbreviations
CAG: coronary angiography
PCI: percutaneous coronary intervention
PWCS: prewire channel stent
SB: side branch
WE: wire exchange
Introduction
Cardiovascular stents are widely used in the treatment of vascular diseases. For example, stents have been implanted in vessels to treat vessel narrowing, occlusion, aneurysm, and dissection, among other conditions1-4. A typical conventional stent system contains a metallic mesh stent that is mounted on a deflated balloon at the distal end of a catheter shaft. The centre of the balloon and its connected catheter shaft contain a channel to inflate or deflate the balloon and another channel through which a guidewire can pass to facilitate movement through the arteries. A catheter with the latter channel is usually designed to function either as a “rapid exchange” catheter or an “over-the-wire” catheter. A catheter in which the proximal port of the channel ends at the catheter shaft near the balloon is called a “rapid exchange” catheter. A catheter in which the proximal port of the channel ends at the proximal end of the catheter is called an “over-the-wire” catheter. However, there is no channel or space between the metallic mesh stent and balloon in conventional stent systems. A guidewire is inserted into the vessel towards the location that needs to be treated. A cardiovascular stent is mounted and carried on a deflated balloon, inserted into the vessel and delivered to the desired location through this guidewire to facilitate movement through the arteries. When the stent is at the desired location, the balloon is inflated and the stent is released.
Vessels have branches; when the vessels are narrowed, the narrowed branches are referred to as “bifurcation lesions”5. When treating a bifurcation lesion with a conventional stent, one guidewire is delivered to the main branch and another guidewire is delivered to a side branch to avoid occlusion of the side branch. After the stent is delivered and deployed at a bifurcation lesion, the guidewire in the main branch should be pulled back and resent into the side branch. This process is achieved through the metallic mesh from the inside lumen of the stent in the vessel and is, by nature, difficult. Thereafter, the guidewire which is in the side branch should be pulled back from the space between the metallic mesh stent and the vessel wall and then resent into the main branch through the lumen of the stent. This procedure is called “wire exchange”6, which is complicated and time-consuming.
A newly designed stent to overcome the shortcomings of a conventional stent is introduced in this study.
Methods
ANIMAL MODEL AND EXPERIMENTAL PROCEDURES
All experimental protocols complied with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and the Animal Care and Use Committees of Xiangtan Central Hospital. Four domesticated medium-sized pigs (39 to 67 kg, aged 10-15 months) were used for all the experiments. The stents were implanted in their coronary arteries as described previously7. In brief, the pigs were made to fast for 12 hours and then put on a liquid diet for four hours before being anaesthetised with ketamine (2 mg/kg), medetomidine (0.06 mg/kg), and azaperone (0.06 mg/kg) by way of an intramuscular injection. Thereafter, the pigs were anaesthetised with propofol (8 mg/kg/min), remifentanil (0.15 μg/kg/min), and cisatracurium (0.1 mg/kg/h) that were injected into an ear vein. The animals were continuously monitored by ECG and made to inhale oxygen (3 L/min). The pigs were laid down on their backs, and their right inguinal region, front chest and limbs were shaved. Then, the right inguinal region was disinfected. The right femoral artery was punctured, and a 6 Fr radial arterial introducer sheath (Terumo Corp., Tokyo, Japan) was placed within it. Unfractionated heparin (50,000 U) was used to avoid thrombus formation. The 6 Fr guiding catheter (Judkins Right 3.5) was advanced to the ostium of the left main coronary artery. The left and right coronary arteries were selectively visualised by coronary angiography (CAG) . Two pairs of branch coronary arteries in a pig were randomly selected for a conventional stent and a prewire channel stent. Two guidewires were sent to the main and side branches separately. For the conventional stent implantation, “wire exchange” should be completed, but for the prewire channel stent implantation, “wire exchange” is not necessary. The time periods of the stent implantations were compared.
STATISTICAL ANALYSIS
Data were reported as means±standard errors of the mean (SEMs), and the experimental and control groups were compared using the Student’s t-test. Differences with p<0.05 were considered statistically significant.
Results
DESIGN OF THE PREWIRE CHANNEL STENT AND WORKING PROCEDURES
Conventional stents usually have a structure that includes a balloon with a metallic mesh material outside it and a central passage with an ancillary component to inflate or deflate the balloon. As shown in Figure 1, the prewire channel stent has an additional second passage positioned between the metallic mesh material and balloon.
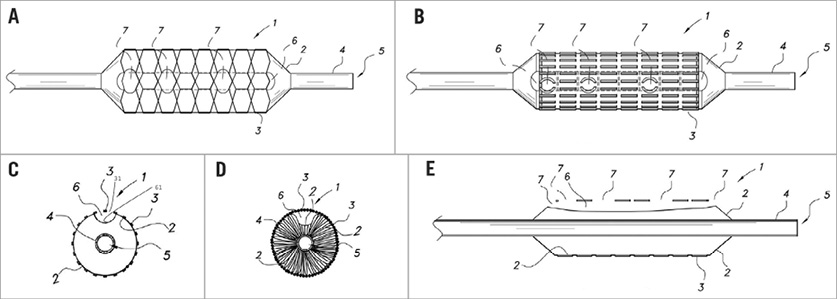
Figure 1. A draft of the prewire channel stent. A) Top elevation view of a prewire channel stent in which a balloon is in an inflated state. B) Top elevation view of the prewire channel stent in panel A in which the balloon is in a deflated state. C) Transverse cross-sectional view of the prewire channel stent as shown in panel A in which the balloon is in an inflated state. D) Transverse cross-sectional view of the prewire channel stent as shown in panel B in which the balloon is in a deflated state. E) Longitudinal cross-sectional view of the prewire channel stent where the balloon is in an inflated state. 1: the prewire channel stent system; 2: balloon; 3: metallic mesh stent; 4: central catheter/first passage; 5: central channel; 6: channel/second passage; 7 openings
The procedures of a conventional stent and prewire channel stent implant for a bifurcation lesion in a vessel have been described previously. In brief, Figure 2A-Figure 2E illustrate a working example of a conventional stent, and Figure 3A-Figure 3E show a working example of a prewire channel stent (balloon catheter and stent system with side guidewire channel).
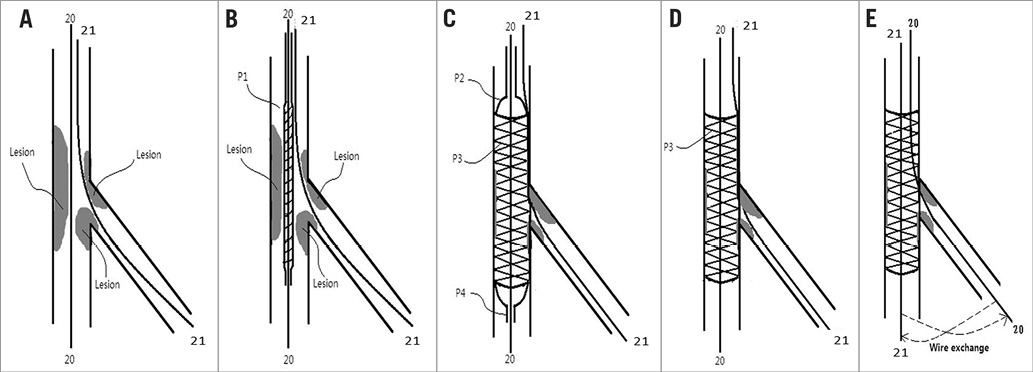
Figure 2. The procedures of a conventional stent implant for a bifurcation lesion in a vessel. A) Step 1. A guidewire (20) is sent to a main branch and a guidewire (21) is sent to a side branch. B) Step 2. A conventional stent and balloon system is sent through the guidewire (20) (by tracking) to the desired position of the vessels. C) Step 3. The balloon is inflated and a conventional stent is dilated to support the vessel; guidewire (20) is still in the main branch through the lumen of the stent, and guidewire (21) is in the side branch through the space between the vessel wall and the metallic mesh material of the stent (i.e., outside the stent). D) Step 4. The balloon is deflated and pulled out of the vessel. Guidewire (20) is still in the main branch through the lumen of the stent, and guidewire (21) is in the side branch through the space between the vessel wall and the metallic mesh material of the stent (i.e., outside the stent). E) Step 5. The guidewire (20) is pulled back and then sent into the lumen of the stent through the metallic mesh to the side branch. The guidewire (21) is also pulled back and sent through the lumen of the stent to the main branch. The process of “wire exchange” is finished as schematically indicated by the dashed lines in panel E.
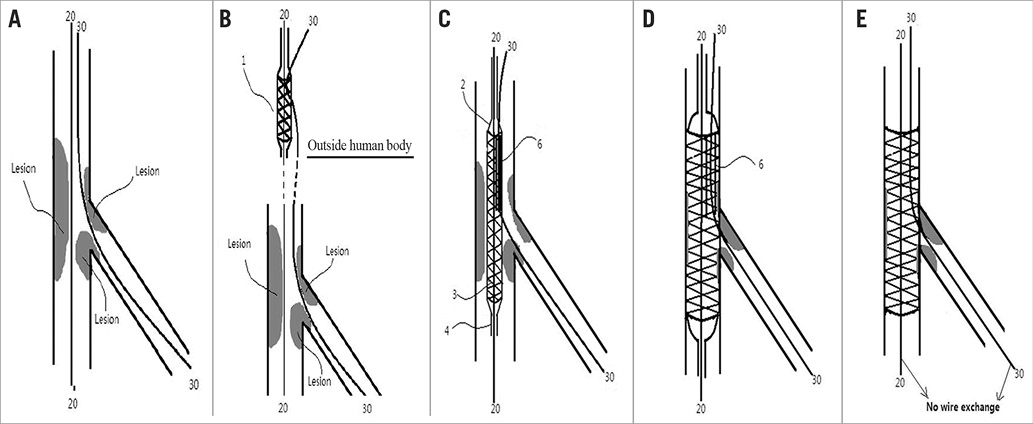
Figure 3. The procedures used for the prewire channel stent implant for a bifurcation lesion in a vessel. A) Step 1. A guidewire (20) is sent to the main branch, and a guidewire (30) is sent to the side branch. B) Step 2. The prewire channel stent with the guidewire (30) goes through the channel or space while outside the human body and is then sent through the guidewire (20) (by tracking with first centralchannel to the desired position of the vessels. C) Step 3. The prewire channel stent with the guidewire (20 and 30) goes through the central channel and side channel or space to the desired position of the vessel (at the bifurcation lesion). The balloon is in a deflated state in this view, and the metallic mesh material is mounted on it, and the guidewire (30) in the lumen of the channel or space (6) and guidewire (20) in the central channel 5. D) Step 4. The balloon (2) is inflated and the metallic mesh material is dilated. The guidewire (30) from the side branch is in the channel or space and the guidewire (20) in the central channel is positioned in the main branch. E) Step 5. The balloon is deflated and withdrawn from the vessel; “wire exchange” is not necessary. 1: the prewire channel stent system; 2: balloon; 3: metallic mesh stent; 4: central catheter/first passage; 5: central channel; 6: channel/second passage
THE PREWIRE CHANNEL STENT IN A PIG MODEL
As shown in Figure 4, the guidewires for main branch and side branch went through the central lumen and second channel of the prewire channel stent and were then sent to the arteries of the heart. As shown in Figure 5, one guidewire was in the main branch and another guidewire in the side branch (Figure 5A). The prewire channel stent was sent to the bifurcation of the vessel (Figure 5B) and was released there with 8 atm pressure before the balloon was pulled back. The “wire exchange” is not necessary for the procedures of the treatment which follow such as balloon dilatation through the stent mesh opening and double kissing balloon technique, etc.
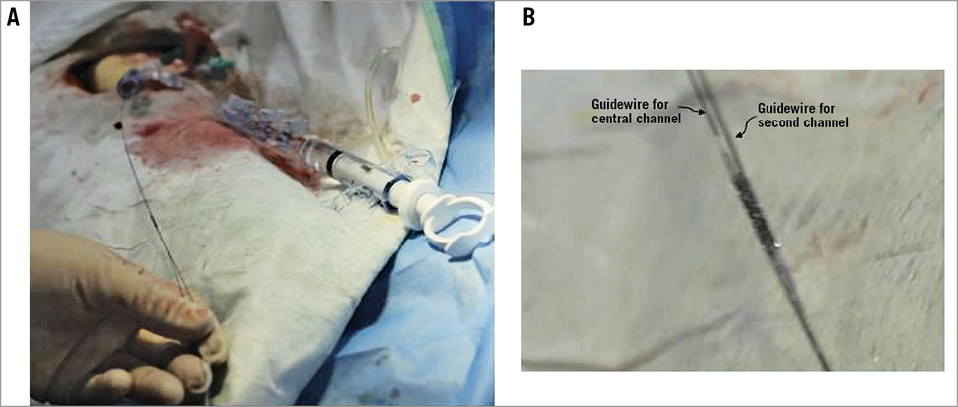
Figure 4. The prewire channel stent working outside the pig model. A) The prewire channel stent being sent to the heart. The guidewire for the side branch of the vessel went through the second channel of the stent, and the guidewire for the main branch of the vessel went through the central channel of the stent outside the pig’s body. Then the stent was sent into the coronary artery. B) Detailed photo of the stent.
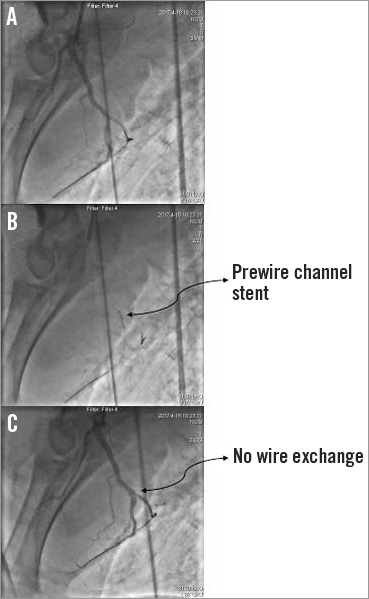
Figure 5. The prewire channel stent working inside the left anterior descending artery (LAD) of the pig model. A) One guidewire was sent to the LAD, while another guidewire was sent to the side branch of the LAD. B) The prewire channel stent was sent to the bifurcation of the LAD. C) The stent was released with 8 atm pressure; “wire exchange” was not necessary.
As shown in Figure 6, another guidewire was sent to another branch of the same pig heart, but the guidewire for the main branch was not moved (Figure 6A). A conventional stent was sent there and released (Figure 6B, Figure 6C). Thereafter, the “wire exchange” had to be finished (Figure 6D) for the procedures which followed.
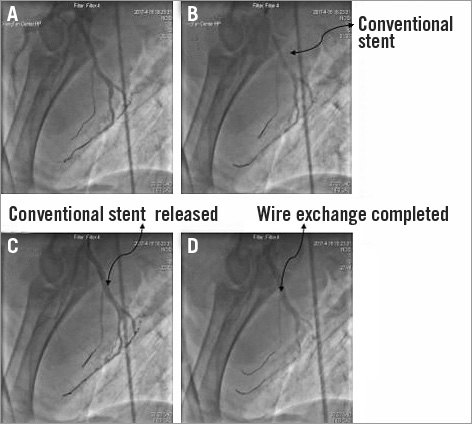
Figure 6. The conventional stent working inside the LAD of the same pig model as in Figure 5. A) One guidewire (same guidewire as in the LAD of Figure 5) was sent to the LAD, and another guidewire was sent to another side branch of the LAD. B) The conventional stent was sent to a bifurcation different from the bifurcation of the LAD in Figure 3. C) The conventional stent was released with 8 atm. D) “Wire exchange” was completed.
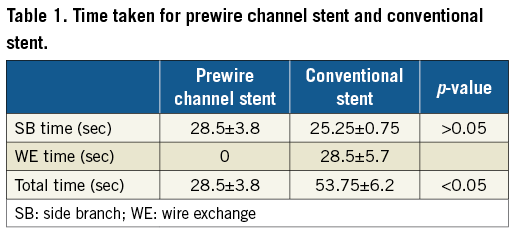
The time taken in percutaneous coronary intervention (PCI) procedures is important and usually related to the level of complication of the procedures. Therefore, we compared the time taken from the point of sending the stent from outside the body to the bifurcation of the vessel ready for further procedures such as balloon dilatation through the stent mesh opening and double kissing balloon technique, etc., in the conventional stent and prewire channel stent groups. Obviously, the time taken in the prewire channel stent group included the time from sending the stent from outside the body to the desired position of the bifurcation of the vessels of the heart, releasing the stent and pulling back the balloon (SB time). However, the time taken in the conventional stent included the time from sending the stent from outside the body to the desired position at the bifurcation of the vessels of the heart, releasing the stent, pulling back the balloon (SB time), wire exchange and pulling back the balloon (WE time). As shown in Table 1, the SB times between the prewire channel stent and conventional stent groups were not different (28.5±3.8 vs. 25.25±0.75 seconds, n=4). However, since the prewire channel stent group did not have “wire exchange”, it did not have WE time, which was 28.5±5.7 seconds in the conventional stent group. The total time spent in the prewire channel stent group was 28.5±3.8 seconds, which is shorter than the 53.75±6.2 seconds (n=4, p<0.05) in the conventional stent group.
Discussion
Bifurcation lesions account for one fourth of all PCIs8,9. Bifurcation interventions tend to have lower rates of procedural success, higher resource utilisation, and higher rates of clinical and angiographic restenosis10,11. Treating bifurcation lesions is one of the most challenging scenarios for an interventional cardiologist because it is not only necessary to recanalise the main vessel but also to avoid SB occlusion. SB occlusion usually occurs in 6-9% of bifurcation stenting10,12. Therefore, a protective guidewire (jailed guidewire) is usually placed in the SB, especially when the SB is very important (e.g., the SB is very large), and the SB may be occluded after the stent is implanted (e.g., an ostial lesion exists in the SB). After the stent implantation, the SB is recrossed by another guidewire (usually the guidewire in the main vessel) from the inside lumen of the stent, which is called “wire exchange”. In this experiment, we compared the time taken between the prewire channel stent and conventional stent systems in normal coronary arteries of a pig model. Two bifurcations of coronary arteries of the same pig were chosen for two stent systems. The result was not surprising as the time taken during the period of sending the stent system to the bifurcation, releasing the stent, and pulling back the balloon was the same for both the prewire channel stent and the conventional stent systems. This means that the prewire channel stent system was as easy to manipulate as the conventional stent system. However, adding the time for “wire exchange”, the time taken for the conventional stent system was much longer than for the prewire channel stent system. Since the bifurcations of the coronary arteries in these experiments were normal (normal coronary arteries of a pig model) and did not have any atheromatous lesions, the “wire exchange” process was much easier to achieve than in the treatment of bifurcation lesions in PCI for a human patient. The “wire exchange” process in PCI is complicated and sometimes difficult to achieve. The jailed guidewire can be a marker for the recrossing guidewire (the most difficult step of “wire exchange”) to increase the chance of recanalising the SB when it is completely occluded because of carina displacement, plaque shift, dissection and haematoma at the SB13,14. However, if the SB is completely occluded by the dissection, the recrossing guidewire may sometimes enter the true lumen of the SB and avoid SB occlusion. If the SB is a small vessel, the patient may have no symptoms or only slight chest pain but, if the SB is a large vessel such as the left circumflex artery, the patient may suffer from acute chest pain, severe haemodynamic impairment, and even risk to life10,15. Most interventional cardiologists are not at ease in this situation. We have designed and investigated a new stent system, the prewire channel stent, which has a second passage positioned between the metallic mesh material and the balloon in addition to a conventional stent in a pig model. This new system can keep the protective guidewire always inside the stent of the main vessel and true lumen of the SB, so that a recrossing guidewire is not necessary. When SB occlusion occurs, the balloon can be easily sent into the SB to rescue the occluded SB and recover the blood flow in the SB.
Limitations
The current work only proved a concept in an animal model. Future studies should elaborate the design of the system. The new system merits clinical trials in humans.
Conclusions
The prewire channel stent with a second channel between the balloon and metal mesh material renders the recrossing guidewire in the SB unnecessary. Moreover, it is also as easily manipulated as a conventional stent.
| Impact on daily practice The prewire channel stent is a new device to treat bifurcation lesions in a more convenient and safer way. |
Acknowledgements
The authors are grateful to APT Medical Inc., Xiangxian, China, for their help in constructing the prewire channel stent system.
Funding
This study was supported by funding provided by the National Natural Science Foundation of China (No: 81170241 to L. Tang, No: 81700294 to G. Lu) and the Technology Project of Xiangtan City (No: SF-YB20161004 to L. Tang).
Conflict of interest statement
L. Tang is the inventor of the prewire channel stent (Pat. No. 201320895986.4, CN; Pat. No. 201510277692.9, CN; Pat. No. 201710826168.1, CN, and 2017211374.6, CN; Pat. No. EP141972935). The other authors have no conflicts of interest to declare.

