
Abstract
Aims: The optimal cut-off value of isolated cardiac biomarker elevation for defining prognostically important percutaneous coronary intervention (PCI)-related myocardial injury is not known. We performed a meta-analysis to evaluate the dose-response relationship between isolated cardiac biomarker elevations and the risk of all-cause mortality following elective PCI.
Methods and results: Twenty-four prospective studies (44,972 patients) were included. Patients with an isolated elevation of cardiac biomarkers had an increased risk of all-cause mortality when compared to those with no elevations (cardiac troponin I: odds ratio [OR] 1.42, 95% confidence interval [CI]: 1.19-1.69; creatine kinase-MB isoenzyme [CK-MB]: OR 1.43, 95% CI: 1.19-1.70). For the dose-response analysis, elevations of cardiac troponin I >3x or CK-MB >1x the 99th percentile upper reference limit (URL) were associated with increased mortality (cardiac troponin I: OR 1.51, 95% CI: 1.05-2.17; CK-MB: OR 1.25, 95% CI: 1.05-1.48). The pooled OR of mortality for each 3xURL increment of cardiac troponin I or CK-MB was 1.33 (95% CI: 1.15-1.53) and 1.38 (95% CI: 1.30-1.47).
Conclusions: We found that a positive dose-response relationship between isolated cardiac troponin I and CK-MB with all-cause mortality and elevated cardiac troponin I >3x or CK-MB >1x the 99th percentile URL was associated with an increased risk of mortality.
Introduction
Elective percutaneous coronary intervention (PCI) is an established treatment for stable coronary artery disease (CAD), accounting for more than three million PCI procedures annually worldwide1,2,3. One of the most common complications following PCI is periprocedural myocardial injury and infarction (also known as type 4a myocardial infarction). Together, these occur in about 35% of elective PCI procedures, and their presence is associated with worse clinical outcomes4,5. According to the fourth universal definition of myocardial infarction (UDMI) in 2018, type 4a myocardial infarction has been defined as an elevation of cardiac troponin values >5x the 99th percentile upper reference limit (URL) in patients with normal baseline values together with electrocardiogram (ECG), angiographic or imaging evidence of new myocardial ischaemia. Procedure-related myocardial injury has been defined as an elevation of cardiac troponin values >1x the 99th percentile URL in patients with normal baseline values together with an ECG6.
In both these definitions, the thresholds for cardiac biomarker elevation have been “arbitrarily chosen”6,7. The difficulty has been in selecting the optimal threshold levels of elevated cardiac biomarkers for defining periprocedural myocardial injury and infarction. This is challenging given the lack of evidence and mixed results from available studies investigating the association between elevated cardiac biomarkers and subsequent long-term adverse clinical outcomes following elective PCI8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31. Therefore, we performed a comprehensive dose-response meta-analysis of all related prospective studies to assess quantitatively the association between elevated post-PCI elevations in cardiac biomarkers (cardiac troponin I, cardiac troponin T, creatine kinase MB isoenzyme [CK-MB]) and adverse clinical outcomes (all-cause mortality, major adverse cardiovascular events [MACE]).
Methods
SEARCH STRATEGY
We report this meta-analysis according to the guidance of the MOOSE (Meta-analysis of Observational Studies in Epidemiology) statement32. We searched PubMed (Medline) and Embase from 1979 to August 2018. We also manually searched the reference lists of the retrieved articles. The keywords used in the search were (creatine kinase or troponin) paired with (angioplasty or stent or PCI or MI).
STUDY OUTCOMES AND SELECTION
The primary endpoint was all-cause mortality. The secondary outcome was MACE. The definition of MACE used by each study included all-cause death, cardiac death, revascularisation or myocardial infarction. These have been listed for each study in Supplementary Table 1.
Inclusion criteria for the retrieved studies were as follows: (1) prospective design; (2) inclusion of outcomes of all-cause mortality and/or MACE; (3) inclusion of multivariable-adjusted or unadjusted relative risk (RR) or odds ratio (OR) and their corresponding 95% confidence intervals (CI), or which provided the number of events and total population in each group; (4) inclusion of different levels of elevated cardiac biomarkers and their related prognosis (to conduct a dose-response meta-analysis, studies with three or more categories of URL were included); and (5) normal cardiac biomarkers at baseline in CAD patients which was defined by individual studies. If the studies provided data for elevated versus non-elevated cardiac biomarkers at baseline, only the non-elevated subgroup was selected.
DATA EXTRACTION
Data were extracted by two independent authors (Y. Li and H. Pei). Discrepancies were resolved by group discussion. The extracted data included source of study (author, publication year, country), population characteristics (mean age, male proportion, percentage of positive cardiac biomarkers, number of subjects and cases, percentage of smoking, diabetes mellitus [DM], hypertension, hyperlipidaemia, previous myocardial infarction, stent implantation and multivessel disease), follow-up period, the different thresholds for cardiac biomarkers, the clinical endpoints, ORs or RRs and their corresponding 95% CI.
STATISTICAL ANALYSIS
We considered the RRs as ORs in the prospective studies. We pooled the ORs from the elevated versus non-elevated categories of cardiac biomarker in each study. If the study did not provide ORs or RRs, we calculated the OR by the number of events and total population in the non-elevated and elevated groups. If different reference categories were reported, we chose the non-elevated category as reference. We pooled the ORs by combining all the categories of elevated biomarker for comparing elevated and non-elevated biomarker categories using the DerSimonian and Laird random-effects model. The random-effects model was also used in the pooled analysis for the potential clinical heterogeneity33. If one study reported multiple categories (≥3 categories), we would calculate the OR using data on the number of cases and non-cases in all of the elevated categories and referent groups. The heterogeneity was assessed by Q statistic, I-squared value and p-value (p<0.05 was considered to be statistically significant). Univariate meta-regression analyses (including follow-up term, gender, area, age, percentage of smoking, DM, hypertension, hyperlipidaemia, previous myocardial infarction, stent implantation, multivessel disease, side branch occlusion) were conducted to explore the potential sources of heterogeneity34. To provide different degrees of elevation of cardiac troponin I and CK-MB in relation to mortality, the degrees of elevated cardiac troponin I were categorised into three standardised intervals (“1 to <3x URL”, “≥3 to <5x URL”, “≥5x URL”) as well as elevated CK-MB into four standardised intervals (“1 to <3x URL”, “≥3 to <5x URL”, “≥5 to <8x URL”, “≥8x URL”). If the study provided the elevated level of cardiac biomarkers by numerical value, we converted this into the corresponding URL according to the upper reference value in each individual study. We separately pooled the ORs of Q-wave MI. We assigned the ORs from each study into standardised intervals according to the range or median of the degrees of elevated cardiac biomarkers in each category. The average URL of elevated biomarkers in each category was estimated by the mean of the lower and upper levels. If the highest category had an open upper level, the mean URL was estimated to be 1.2x the level of the lower levels35. The weighted linear regression model was used to explore the dose-response relationship between elevated cardiac troponin I and CK-MB and the risk of mortality .
All data analyses were performed using Stata software, version 10.0 (StataCorp., College Station, TX, USA) and RevMan software, version 5.0 (Cochrane Collaboration, Oxford, United Kingdom).
Results
SEARCH RESULTS
Twenty-four prospective studies involving 43,582 subjects were included in our meta-analysis; the selection process is shown in Figure 1. The data of studies published by Novack et al16 and Lindsey et al36 were derived from the same EVENT registry. The former provided the data on cardiac troponin I and CK-MB and the latter detailed data on CK-MB36. To avoid duplication, we only extracted data related to cardiac troponin I in the study by Novack et al16 and CK-MB in the study by Lindsey et al36.
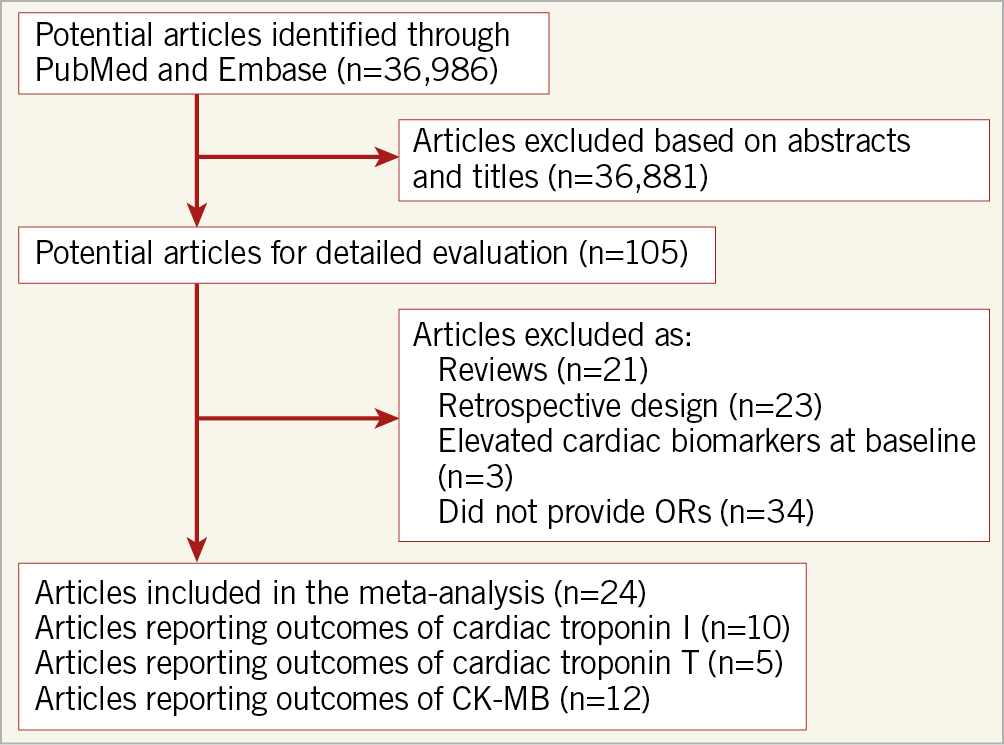
Figure 1. Flow chart of the trial selection process. CK-MB: creatine kinase myocardial band isoenzyme; OR: odds ratio
STUDY CHARACTERISTICS
Supplementary Table 1 shows the main characteristics of the data extracted from the included studies. The follow-up time ranged from 3 months to 5.6 years with a median value of 12 months.
CARDIAC TROPONIN I AND ALL-CAUSE MORTALITY
Compared with the group with non-elevated cardiac troponin I levels post PCI, the group with elevated ones was associated with an increased risk of all-cause mortality (11 studies, 13,932 subjects, OR 1.42, 95% CI: 1.19 to 1.69, p=0.000, I2=12.1%, p for heterogeneity 0.33) (Figure 2). In univariable meta-regression, none of the variables was related to risk of death (Supplementary Table 2).
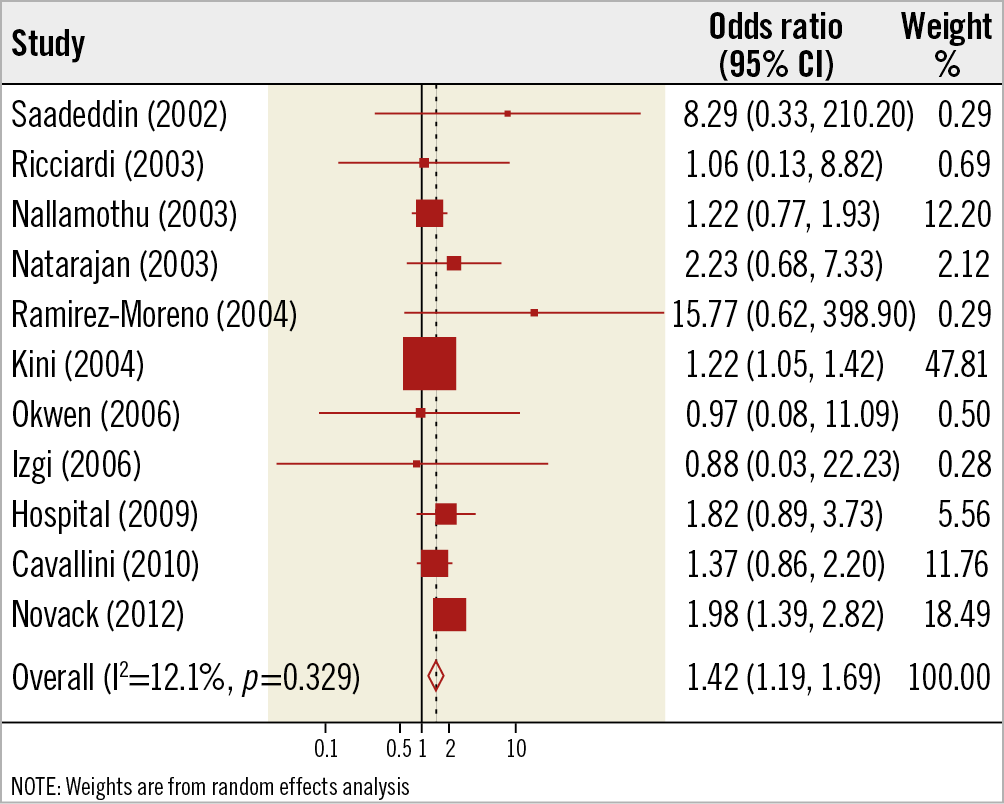
Figure 2. Elevated versus non-elevated cardiac troponin I levels and risk of all-cause mortality
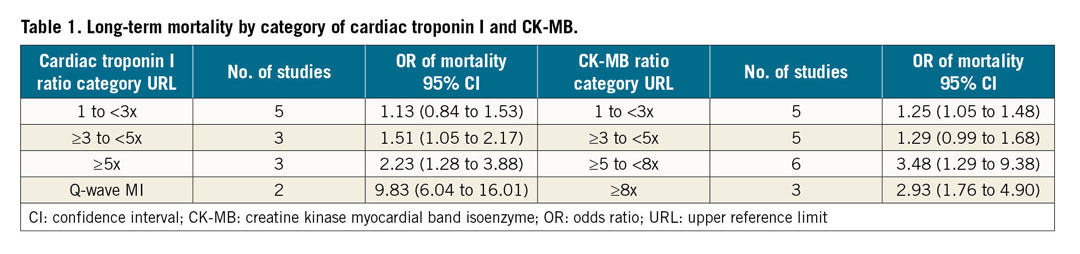
Furthermore, an elevated cardiac troponin I >3-5x the 99th percentile URL was associated with an increased risk of all-cause mortality (OR 1.51, 95% CI: 1.05 to 2.17, p=0.025) (Table 1). The dose-response analysis showed that, for every 3x the 99th percentile URL increment in cardiac troponin I elevation, the pooled OR was 1.33 (95% CI: 1.15 to 1.53, p=0.000) for the risk of all-cause mortality in patients undergoing elective PCI.
CK-MB AND ALL-CAUSE MORTALITY
Compared with the group with non-elevated CK-MB post PCI, patients with an elevated one were associated with an increased risk of all-cause mortality (7 studies, 27,486 subjects, OR 1.43, 95% CI: 1.19 to 1.70, p=0.000, I2=54.6%, p for heterogeneity 0.04) (Figure 3). In univariable meta-regression, none of the variables was related to risk of death (Supplementary Table 3).
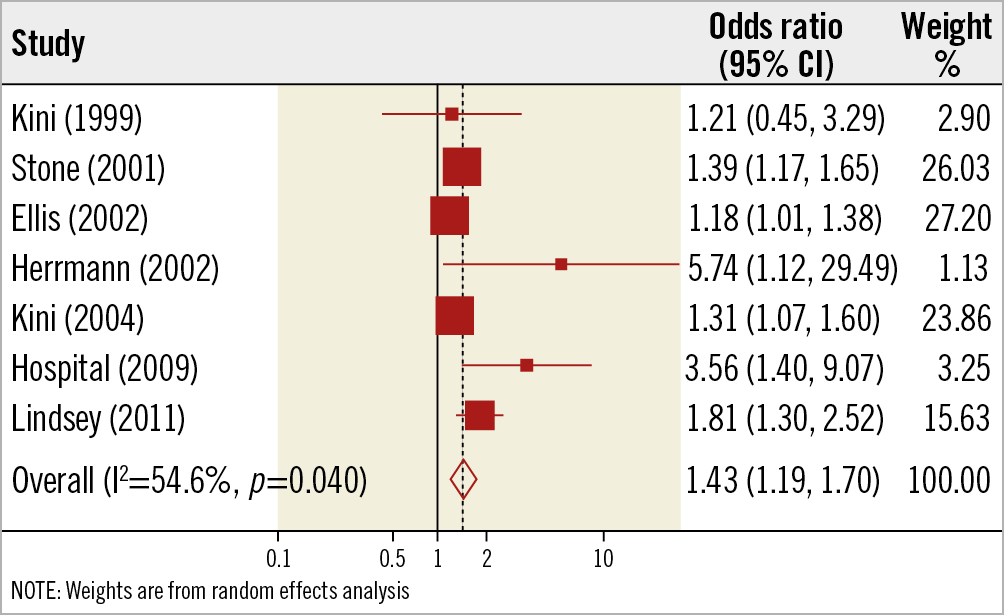
Figure 3. Elevated versus non-elevated CK-MB levels and risk of all-cause mortality.
Furthermore, for different categories of elevation in CK-MB, a rise of 1-3x the 99th percentile URL or more was associated with all-cause mortality (OR 1.25, 95% CI: 1.05 to 1.48 for the category of 1-3x the URL, p=0.014) (Table 1). The dose-response analysis showed that, for every 3x the 99th percentile URL increment in CK-MB elevation, the pooled OR was 1.38 (95% CI: 1.30 to 1.47, p=0.000) for the risk of mortality in patients undergoing elective PCI (Figure 4).
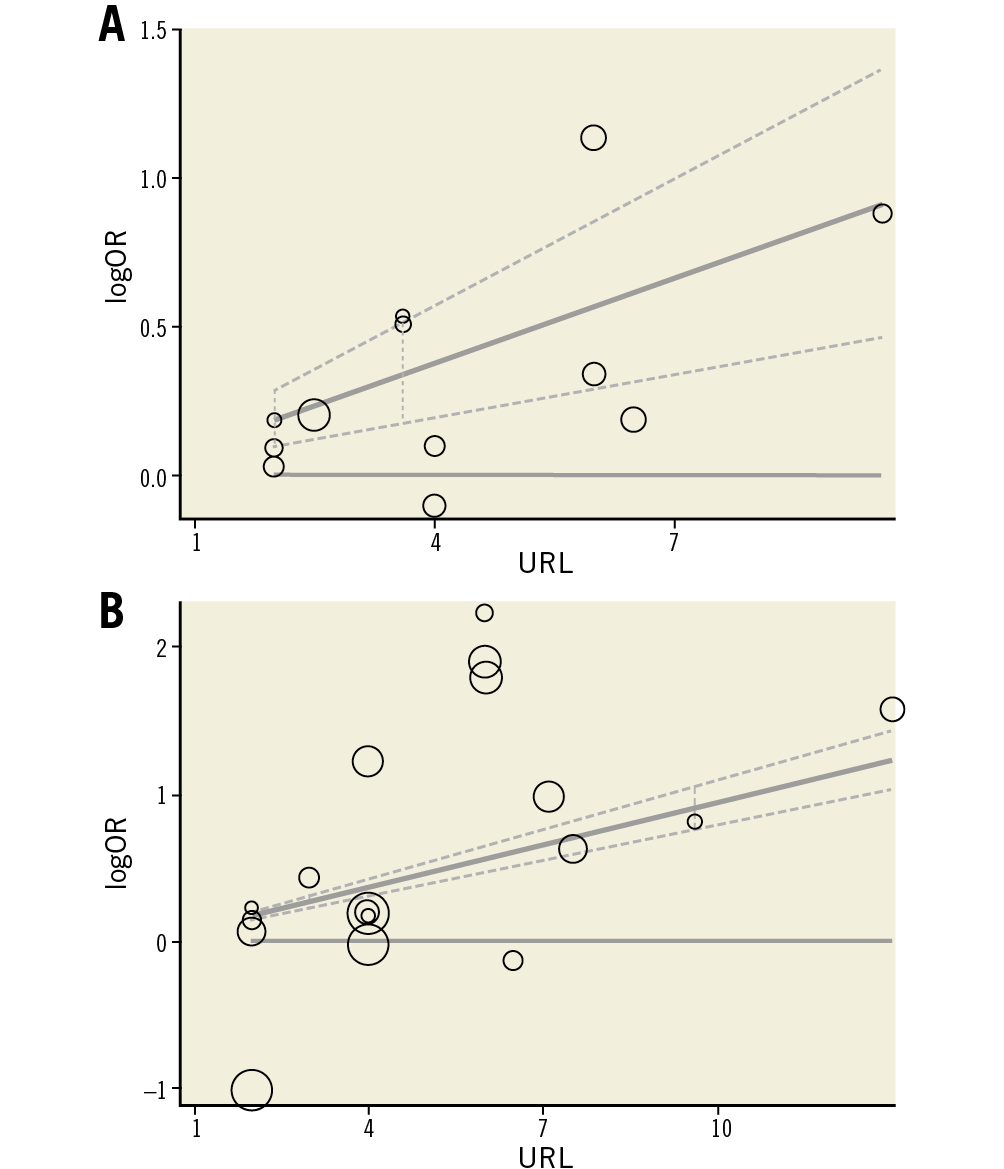
Figure 4. Dose-response relationship for cardiac troponin I (A) or CK-MB (B) and risk of all-cause mortality. Each small black circle indicates logOR for each category of cardiac troponin I level which is proportional to its statistical weight; solid line represents weighted logOR, and its two accompanying dashed lines represent its lower and upper CIs. Horizontal solid line indicates the null hypothesis (logOR=0). CI: confidence interval; CK-MB: creatine kinase myocardial band isoenzyme; OR: odds ratio
ELEVATED CARDIAC BIOMARKERS AND MACE
Compared with the non-elevated group, patients with elevated cardiac troponin I, cardiac troponin T and CK-MB levels were associated with an increased risk of MACE (Supplementary Figure 1-Supplementary Figure 3).
Discussion
In this comprehensive dose-response meta-analysis of prospective studies, we found that elevated levels of cardiac troponin I and CK-MB post PCI were all associated with a risk of all-cause mortality in patients undergoing elective PCI. More importantly, >3x the 99th percentile for cardiac troponin I or >1x the 99th percentile URL for CK-MB was associated with an increased risk of mortality. Crucially, mortality increased by 33% or 27% for each increment of 3x the 99th percentile URL for cardiac troponin I or CK-MB levels post PCI, respectively.
Previous studies have provided conflicting results with regard to the relationship between elevated cardiac troponin levels post PCI and long-term prognosis10,11,16,20,30. Previous meta-analyses including both retrospective and prospective studies have found a positive association between cardiac troponin and adverse events4. To avoid selection and recall bias, we only pooled all prospective studies and found a positive association between elevated cardiac troponin I and all-cause mortality or MACE, as well as elevated cardiac troponin T and MACE. Moreover, our meta-analysis has provided new insights. Furthermore, our meta-analysis has shown that cardiac troponin I >3x the 99th percentile URL post PCI was associated with an increased risk of death whereas an elevation of 1-3x the URL was not associated with mortality. Periprocedural myocardial injury/infarction, as an important procedural safety endpoint, has been highlighted for its high incidence and predictive value. Current ACCF/AHA/SCAI guidelines for PCI have indicated a class II-b recommendation for routine measurement of cardiac biomarkers37. Results from our meta-analysis showed that, for each 3x the 99th percentile URL increment of cardiac troponin I, the risk of mortality was increased by 33%. Therefore, it would be helpful to risk-stratify patients post PCI by routine screening for cardiac troponin I within 24 hours. Our meta-analysis has indicated that a post-procedural rise in cardiac troponin T was related to the risk of MACE. Nevertheless, large-scale prospective trials are needed to confirm the relationship between post-procedural elevation in cardiac troponin T and prognosis, especially for the hard endpoints such as all-cause mortality.
Previous studies on the association between elevated CK-MB levels and adverse prognosis have also reported mixed findings. Consistent with previous meta-analyses, we combined all the prospective trials finding a positive association between any elevation in CK-MB and all-cause mortality or MACE38,39. Given that cardiac troponin is more sensitive and specific than CK-MB in detecting cardiac injury and infarction, the latter has not been included in the latest universal definition criteria for MI to define type 4a6. Our meta-analysis has provided evidence that any elevation of CK-MB post PCI could predict an increased risk of death. Moreover, there was a linear dose-response relationship between CK-MB elevation and all-cause mortality. Each increment of 3x the 99th percentile URL in CK-MB levels increased mortality by 27%. The results of our meta-analysis have provided evidence that CK-MB may still play a role in risk stratification in patients undergoing PCI where cardiac troponin levels are not available.
Patients undergoing complex PCI (such as chronic total occlusions [CTOs]40 or multivessel PCI41) are at increased risk of sustaining periprocedural myocardial injury/infarction, compared to simple PCI. As such, whether these patients should be treated with optimal medical therapy instead of revascularisation by PCI is not clear. The results from the recently reported ISCHEMIA trial failed to show that routine invasive therapy with PCI was associated with a reduction in major adverse ischaemic events, when compared with optimal medical therapy, among stable patients with moderate ischaemia (Hochman JS, Spertus JA. International Study of Comparative Health Effectiveness With Medical and Invasive Approaches – ISCHEMIA. Presented at AHA 2019, Philadelphia, PA, USA, 16 November 2019). These findings suggest that, at least in this patient group, optimal medical therapy should be considered first before proceeding to PCI. Whether this approach should also be applied to higher-risk PCI patients with CTO or multivessel disease is not clear and needs to be determined.
Limitations
We excluded some studies that did not separately report the data of cardiac troponin I, cardiac troponin T and CK-MB; therefore, the unpooled data might have affected the results. Not all of the included trials provided the multivariable adjusted OR, and the residual confounders were not negligible. Stand-alone or isolated elevations in cardiac biomarkers point to a diagnosis of PCI-related myocardial injury but are insufficient for a diagnosis of type 4a myocardial infarction, for which additional evidence of myocardial ischaemia and angiographic complications as defined in the fourth UDMI is required. We could not perform a multiple meta-regression covering all population characteristics, because only three included trials provided all of the variables for cardiac troponin I along with three trials for CK-MB. We cannot rule out a potential publication bias for our analyses. However, a test of publication bias was not performed for the problematic issue of when heterogeneity is substantial. A number of studies have reported elevations in baseline (pre-PCI) cardiac troponin to be strong predictors of clinical outcomes42,43,44. We undertook a subgroup analysis of the four studies which only included patients with baseline cardiac troponin I <99th percentile URL (Supplementary Figure 4). Interestingly, we found that post-PCI elevations in cardiac troponin I did not predict mortality. Only one study used troponin T with an actual 99th percentile URL. This subgroup analysis was likely to be underpowered due to the limited number of studies and patients, and a larger patient-level meta-analysis would be more meaningful. Finally, our meta-analysis used pooled data rather than individual data, which restricted detailed analysis for potential confounding factors such as angiographic, procedural (e.g., use of calcium-modifying adjuncts), patient characteristics, and follow-up period. Therefore, future studies with a prospective design, large sample size and hard endpoints are needed for further investigation into cardiac troponin and CK-MB.
Conclusions
Our comprehensive dose-response meta-analysis of 24 prospective studies comprising 44,972 patients has demonstrated that elevated levels of cardiac troponin I or CK-MB predict all-cause mortality following elective PCI. We provide evidence that an optimal cut-off elevation of cardiac troponin I >3x or CK-MB >1x the 99th percentile URL relates to an increased risk of mortality. Each increment of 3x the 99th percentile URL in cardiac troponin I and CK-MB was associated with an increased mortality of 33% and 27%, respectively. Future research aimed at preventing or reducing the development of PCI-related myocardial injury/infarction is warranted.
|
Impact on daily practice Patients with elevated levels of cardiac troponin I (a cut-off value of cardiac troponin I >3x the 99th percentile URL) or CK-MB (a cut-off value of CK-MB >1x the 99th percentile URL) may have a mortality risk following elective PCI. In addition, each increment of 3x the 99th percentile URL in cardiac troponin I and CK-MB was associated with an increased mortality of 33% and 27%, respectively. Using this cut-off value, patients at higher risk of MACE and death can be identified and may benefit from prolonged hospital stay, closer monitoring, more intensified treatment, or more intensive outpatient follow-up to improve outcomes. Therefore, our findings suggest that cardiac biomarkers should be routinely measured post PCI. |
Funding
This work was supported by the National Natural Science Foundation of China (numbers 81970290, 81760096, and 81400271) and the Inner Mongolia Natural Science Projects (number 2015MS0858). D.J. Hausenloy is supported by the Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021) and the EU-CARDIOPROTECTION CA16225 Cooperation in Science and Technology (COST) Action.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

