Abstract
Background: Creatinine clearance (CrCl) is an independent determinant of mortality in predictive models of revascularisation outcomes for complex coronary artery disease.
Aims: This study aimed to investigate the impact of preprocedural biological markers on 10-year mortality following coronary revascularisation.
Methods: The SYNTAX Extended Survival (SYNTAXES) study evaluated the 10-year vital status follow-up of 1,800 patients with de novo three-vessel (3VD) and/or left main coronary artery disease (LMCAD) randomised to include percutaneous or surgical coronary revascularisation. The associations between mortality and preprocedural C-reactive protein (CRP), haemoglobin, HbA1c, CrCl, fasting triglycerides, low-density lipoprotein cholesterol, and high-density lipoprotein cholesterol were analysed.
Results: Out of 1,800 patients, 460 patients died before the 10-year follow-up. CRP, HbA1c and CrCl with threshold values of ≥2 mg/L, ≥6% (42 mmol/mol) and <60 ml/min, respectively, were associated with 10-year all-cause death (adjusted hazard ratio [95% confidence interval]: 1.35 [1.01-1.82], 1.51 [1.16-1.95], and 1.46 [1.07-2.00], respectively). There was no significant interaction between the biological markers on all-cause mortality and the type of revascularisation. Preprocedural lipid markers were not significantly associated with 10-year all-cause death, but the non-use of statins was a determinant factor of worse prognosis (adjusted hazard ratio [95% confidence interval]: 1.68 [1.26-2.25]).
Conclusions: Preprocedural biomarkers, such as CRP and HbA1c, are associated with long-term mortality post revascularisation, regardless of the revascularisation technique. Conventional lipidic biomarkers associated with high-risk of cardiovascular events seem to be effectively mitigated by the long-term use of statins, whereas the non-use of statins was a factor of a worse prognosis, emphasising the importance of pharmacological treatment. Trial registration: SYNTAXES ClinicalTrials.gov: NCT03417050. SYNTAX ClinicalTrials.gov: NCT00114972.
Introduction
Decision making for treatment in patients with complex coronary artery disease (CAD) should be based on the Heart Team’s discussion and active patient participation. We previously developed the SYNTAX score II to predict 4-year mortality and the SYNTAX score II 2020 to predict both 10-year mortality and 5-year major adverse cardiovascular events, defined as a composite of all-cause death, non-fatal stroke, or non-fatal myocardial infarction (MI)12. These scores can be used in individual patients given percutaneous coronary intervention (PCI) or coronary artery bypass grafting surgery (CABG), to support decision making based on the anatomical complexity of CAD and comorbidities. A preprocedural biological marker, creatinine clearance (CrCl) was incorporated into these scores. Only the impact of CrCl was evaluated in the development of the SYNTAX score II 2020, although preprocedural biological markers, such as haemoglobin, white blood cells (WBC), C-reactive protein (CRP), and brain natriuretic peptide have been reported in the literature to be associated with outcomes after revascularisation3456. Of note, the logistic clinical SYNTAX score was developed, validated and updated to enable a more accurate prediction of mortality after PCI; the incorporation of biological markers (haemoglobin and WBC) has improved the predictivity789.
This study aimed to investigate the impact of preprocedural biological markers on 10-year all-cause mortality in the SYNTAXES trial in patients with three-vessel disease (3VD) and/or left main coronary artery disease (LMCAD)10. We also investigated whether preprocedural biological markers that are significantly associated with 10-year mortality could improve the predictivity of the SYNTAX score II 2020.
Methods
STUDY DESIGN AND PARTICIPANTS
The design and results of the 5-year follow-up of the SYNTAX trial (NCT00114972) have been previously reported11. The 10-year vital status has been reported in the SYNTAXES study (NCT03417050)10. The SYNTAX trial was a prospective, multicentre, international randomised controlled trial. Patients with de novo 3VD and/or LMCAD, who were anticipated to achieve a clinical equipoise between CABG and PCI based on clinical assessment and the consensus of a Heart Team, were enrolled and randomised in a 1:1 fashion either to receive PCI (n=903) with TAXUS Express paclitaxel drug-eluting stents (Boston Scientific) or CABG (n=897) between March 2005 and April 2007. The ethics committee at each investigating centre approved the trial and all participants provided their written informed consent prior to enrolment. Follow-up complied with local law, regulations of each institution and the Declaration of Helsinki.
ENDPOINTS AND DEFINITIONS
Before revascularisation, blood samples for assessment of CRP, haemoglobin and haematocrit, HbA1c and fasting glucose, serum creatinine, fasting total cholesterol, triglycerides (TG), and high-density lipoprotein cholesterol (HDL-C), gamma-glutamyl transferase (GGT), alanine aminotransferase (ALT), and creatine kinase (CK)/CK-MB were analysed by an independent central chemistry laboratory (Labcorp Drug Development, Geneva, Switzerland, and Indianapolis, IN, USA). In the SYNTAX trial, a detailed drug history was collected at discharge, as well as at the 1-month, 6-month, 1-year, 3-year and 5-year follow-ups12, and medication status was treated as a time-varying covariate. In the SYNTAXES study, vital status at 10 years was confirmed by contact with medical care personnel or using electronic healthcare record review and national death registries. Patients with missing vital status were included in the analysis but censored if lost to follow-up. Five patients in two institutes that did not participate in the SYNTAXES study for the 10-year extended follow-up were censored at five years10.
STATISTICAL ANALYSIS
Primary analyses were carried out on the following seven biomarkers as predictors: 1) CRP, 2) haemoglobin, 3) HbA1c, 4) creatinine clearance (CrCl) according to the Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI) equation, 5) TG, 6) low-density lipoprotein cholesterol (LDL-C) calculated by the Friedewald Equation, and 7) HDL-C. Based on the World Health Organization (WHO) definition of anaemia and comparison between haemoglobin and haematocrit (Supplementary Table 1A), we selected haemoglobin for the primary analysis. We selected HbA1c, a maker of glycaemic status as being more relevant than fasting glucose (Supplementary Table 1B). CK, GGT and ALT were used for the assessment of periprocedural myocardial infarction and liver function in the SYNTAX trial and were not associated with 10-year all-cause mortality (Supplementary Table 1C). The changes in hazard for all-cause mortality in the selected seven biomarkers were modelled using restricted cubic spline functions (3 knots) in an adjusted Cox regression model. Hazard ratios (HRs) with 95% confidence intervals (CIs) for all-cause mortality were determined on the basis of Cox proportional hazards regression, unadjusted and adjusted for the following baseline variables: age, current smokers, peripheral vascular disease, chronic obstructive pulmonary disease, left ventricular ejection fraction, CrCl, disease type (LMCAD or 3VD), and anatomical SYNTAX score2. Treatment interaction (PCI and CABG) was evaluated to support decision making.
Additionally, we compared the survival in patient groups defined by cut-off values of each biomarker. Moderate or severe anaemia was defined by World Health Organization criteria (haemoglobin <11 g/dl). The following binary cut-off levels were selected according to prior studies; CRP, HbA1c, CrCl, TG, LDL-C, and HDL-C were 2 mg/L13, 6.0% (42 mmol/mol) in the overall population141516, 60 ml/min17, 150 mg/dl18, 70 mg/dl13, and 40 mg/dl in males or 50 mg/dl in females18, respectively. In the analyses of non-medically treated diabetes and medically treated diabetes, differential cut-off values of HbA1c (6.0% [42 mmol/mol] and 8.0% [64 mmol/mol], respectively) were applied according to the results of a previous meta-analysis and research1516. The following equation was applied for the conversion from the National Glycohemoglobin Standardization Program (NGSP) unit to the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) unit: IFCC (mmol/mol)=10.93×NGSP (%)–23.50. The association between statin or diabetes therapy and all-cause mortality was also evaluated. The cumulative incidence of all-cause death was calculated using the Kaplan-Meier method. Kaplan-Meier survival curves were analysed using the log-rank test.
To investigate whether preprocedural biological markers could improve the predictivity of the SYNTAX score II 2020, we incorporated biological markers that were significantly associated with 10-year mortality into the SYNTAX score II 2020. For the updated SYNTAX score II 2020 including biological markers, multiple imputations (20 times) of missing values were used to make efficient use of the available data, and the same methods of the score development and validation were applied as for the development of the original SYNTAX score II 20202.
Continuous variables were expressed as mean (standard deviation) and were compared using the Student's t-test. Categorical variables were reported as percentages (numbers) and were compared using the chi-square test. A two-sided p-value <0.05 was considered statistically significant. Analyses were performed using SPSS Statistics, version 26 (IBM), Stata 15 (StataCorp) and R version 3.6.0 (R Foundation for Statistical Computing).
Results
STUDY POPULATION AND VALUES OF BIOLOGICAL MARKERS
A 10-year follow-up was obtained for 1,689 (93.8%) patients and the completeness of the follow-up differed according to country (Supplementary Table 2). The median (interquartile range) of follow-up for 111 patients who did not complete the 10-year follow-up was 5.02 (4.96-5.47) years. Out of 1,800 patients, CRP, haemoglobin, HbA1c, CrCl, TG, LDL-C, and HDL-C values were available in 1,648 (91.6%), 1,550 (86.1%), 1,557 (86.5%), 1,638 (91.0%), 1,638 (91.0%), 1,566 (87.0%) and 1,588 (88.2%) patients, respectively. The distribution of these values is shown in Figure 1.
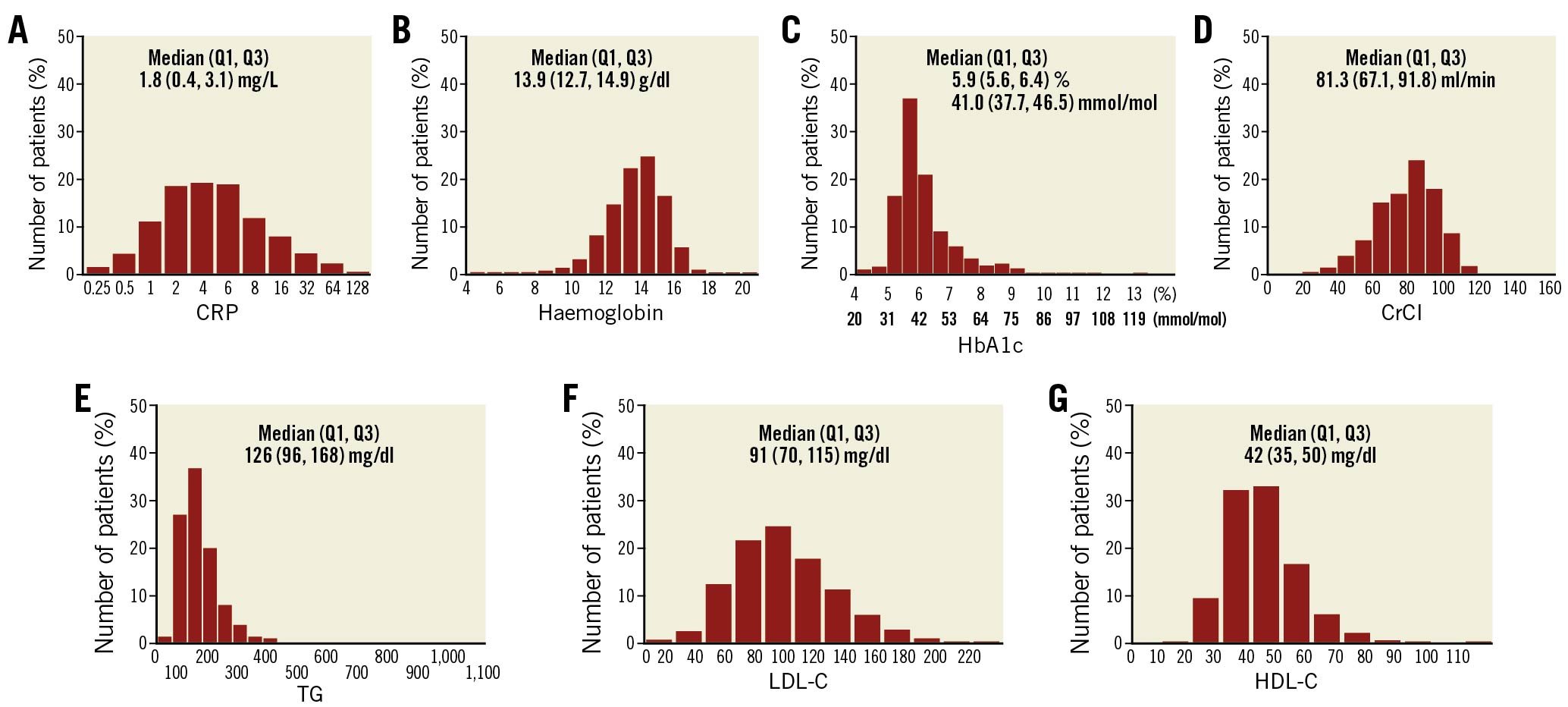
Figure 1. Distribution of biological markers. Distribution of (A) C-reactive protein (CRP), (B) haemoglobin, (C) HbA1c, (D) creatinine clearance (CrCl), (E) triglycerides (TG), (F) low-density lipoprotein cholesterol (LDL-C), and (G) high-density lipoprotein cholesterol (HDL-C) values. Q1: quartile 1; Q3: quartile 3.
IMPACT OF BIOLOGICAL MARKERS ON ALL-CAUSE MORTALITY
The preprocedural biological marker values of patients were compared between patients alive or dead at the 10-year follow-up (Table 1). Patients who died before 10 years had higher CRP and HbA1c, and lower haemoglobin and creatinine clearance compared to patients who were still alive at the 10-year follow-up. The association between biological marker values and mortality are shown with cubic spline curves (Figure 2). After adjustment, CRP (10 mg/L increase), haemoglobin (1 g/dl decrease) and HbA1c (1% [11 mmol/mol] increase) were significantly associated with 10-year all-cause death (adjusted HR [95% CI]: 1.07 [1.01-1.13], 1.08 [1.01-1.17], and 1.15 [1.02-1.30], respectively). When the values were treated as categorical variables, CRP ≥2 mg/L and HbA1c ≥6% (42 mmol/mol) were also significantly associated with 10-year all-cause death in the overall population (adjusted HR [95% CI]: 1.35 [1.01-1.82] and 1.51 [1.16-1.95], respectively, Figure 3). CKD defined as CrCl <60 ml/min had significant impact on 10-year mortality (adjusted HR [95% CI]: 1.46 [1.07-2.00]). On the other hand, lipid markers, such as TG, LDL-C and HDL-C, were not statistically associated with 10-year all-cause death. There was no evidence of any interaction in the association of biological markers and all-cause mortality between the PCI and CABG groups (Supplementary Figure 1, Supplementary Figure 2).
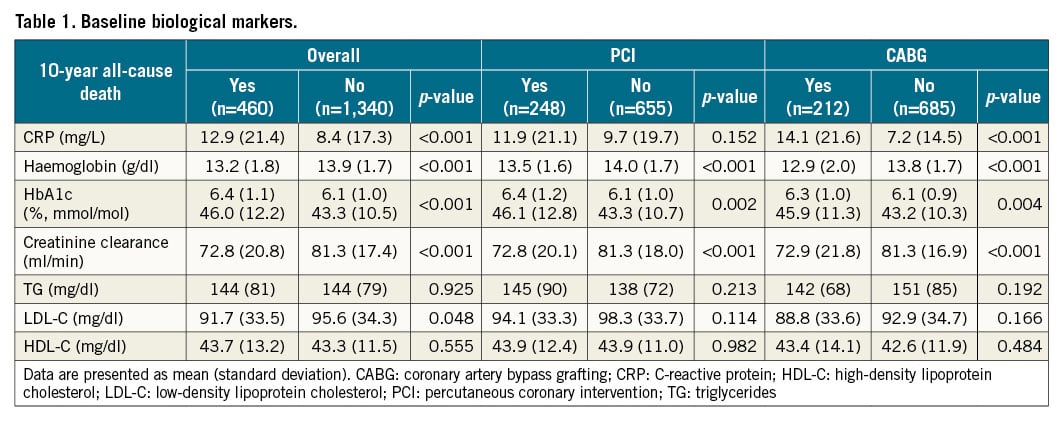
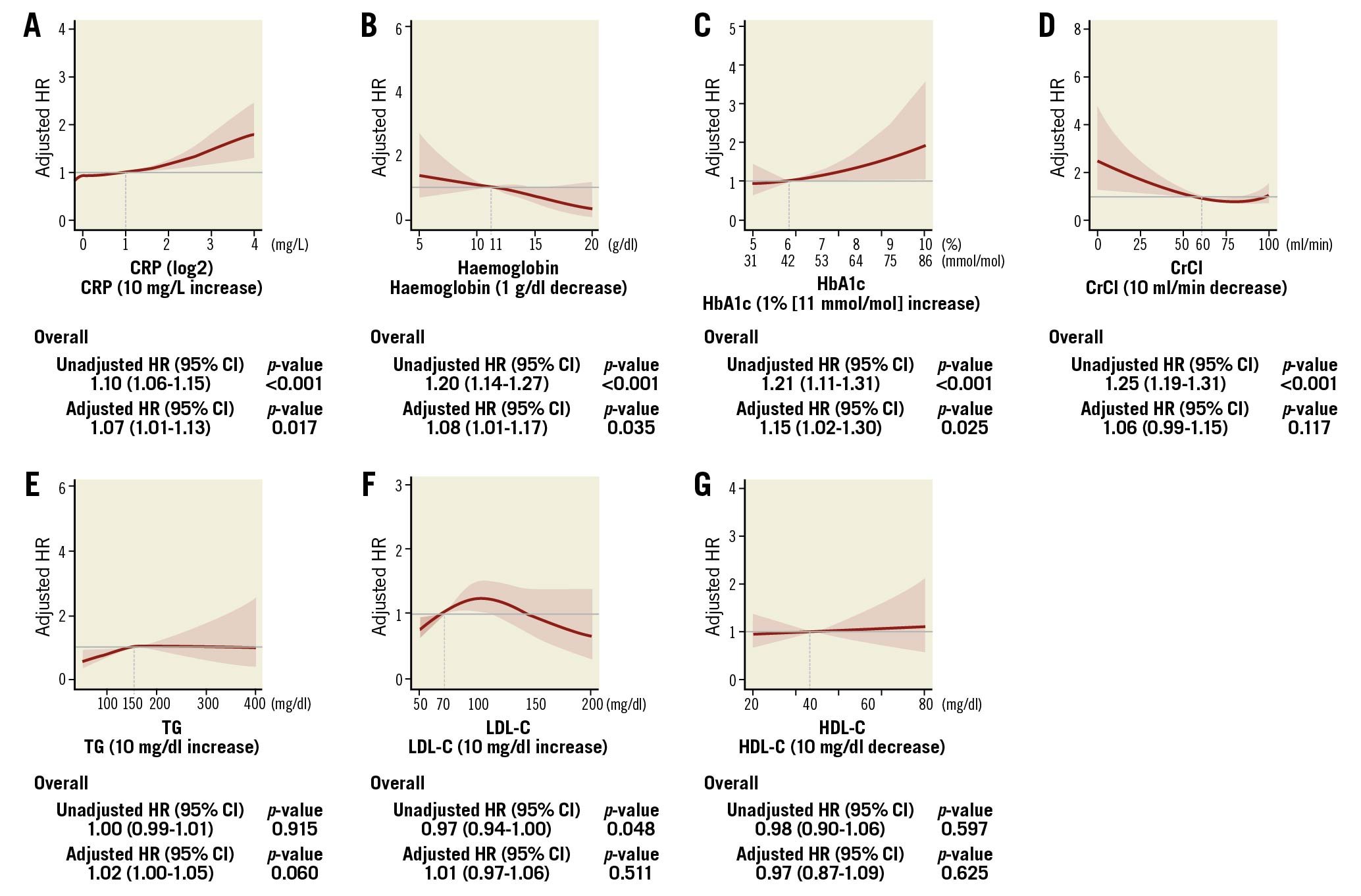
Figure 2. Impact of biological markers on 10-year mortality in the overall population. The red curve with the light red area indicates the adjusted hazard ratio (HR) with 95% confidence interval (CI) for 10-year mortality according to (A) CRP with reference of 2 mg/L, (B) haemoglobin with reference of 11 g/dl, (C) HbA1c with reference of 6.0% (42 mmol/mol), (D) CrCl with reference of 60 ml/min, (E) TG with reference of 150 mg/dl, (F) LDL-C with reference of 70 mg/dl, and (G) HDL-C with reference of 40 mg/dl in male or 50 mg/dl in female. The number of knots for each cubic spline curve was three. CrCl: creatinine clearance; CRP: C-reactive protein; HDL-C: high-density lipoprotein cholesterol; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides
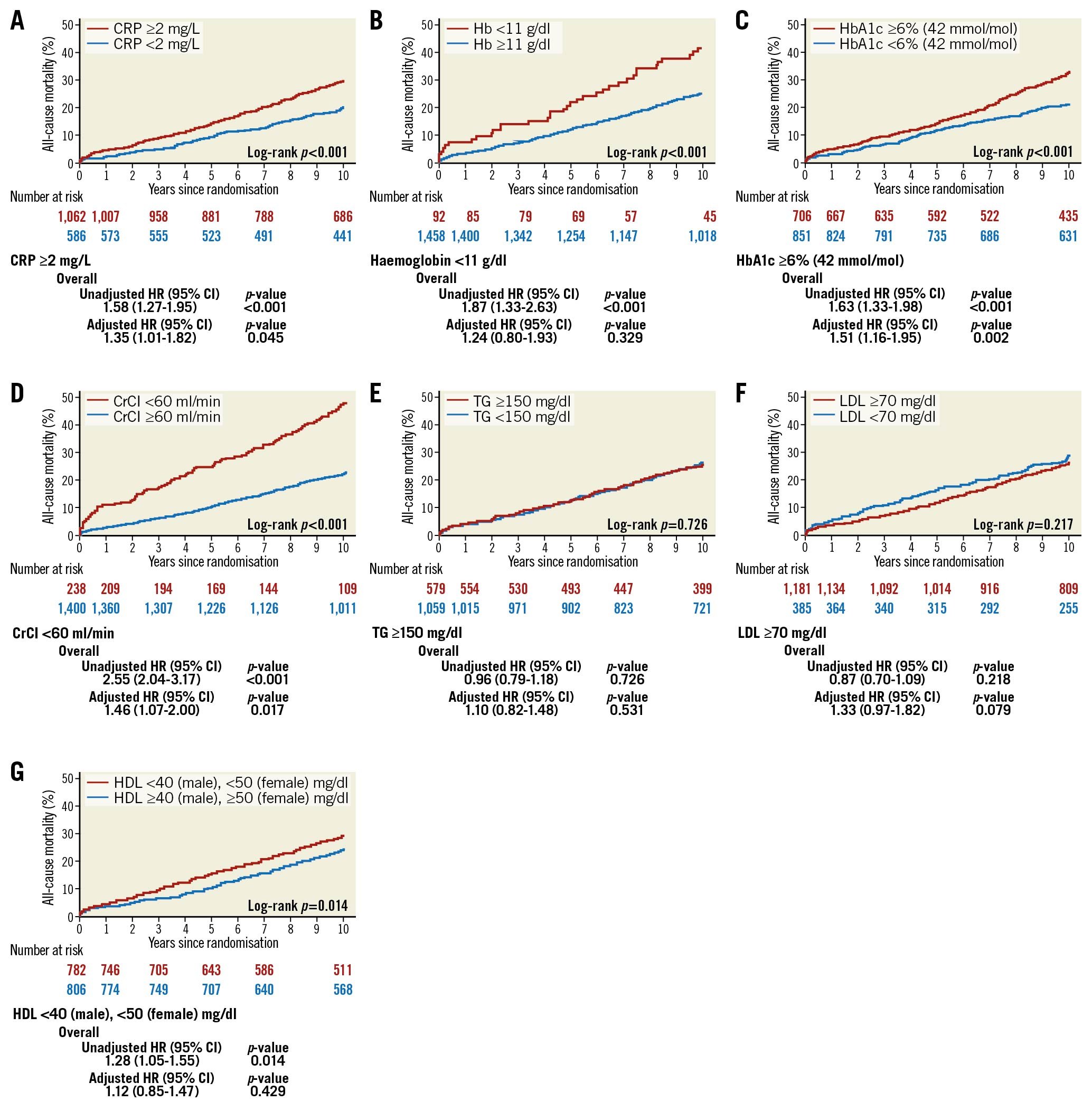
Figure 3. Kaplan-Meier curves for all-cause mortality at 10 years in the overall population. Mortality rates according to (A) CRP, (B) haemoglobin, (C) HbA1c, (D) CrCl, (E) TG, (F) LDL-C, and (G) HDL-C. HR with 95% CI of each categorical variable for 10-year mortality is shown in the lower panel. CI: confidence interval; CrCl: creatinine clearance; CRP: C-reactive protein; HDL-C: high-density lipoprotein cholesterol; HR: hazard ratio; LDL-C: low-density lipoprotein cholesterol; TG: triglycerides
DIABETES AND HbA1c
In the overall population, increases in preprocedural HbA1c were significantly associated with an increased hazard of all-cause death over 10 years (Figure 2, Figure 3). Patients without diabetes at enrolment and with HbA1c ≥6% (42 mmol/mol) had a higher rate of mortality at 10 years (adjusted HR [95% CI]: 1.38 [1.00-1.90]), especially after PCI (adjusted HR [95% CI]: 1.82 [1.18-2.80]; Supplementary Figure 3). In contrast, in patients with medically treated diabetes at enrolment, preprocedural HbA1c did not predict the 10-year all-cause mortality (Supplementary Figure 3, Supplementary Figure 4). Their medication adherence was 87.0% at discharge and 78.7% at five years (Supplementary Table 3). Diabetic patients with interrupted pharmacological treatment had a higher mortality rate, compared to those treated with diabetes medication (39.0% vs 31.5%, adjusted HR [95% CI]: 1.66 [1.00-2.75], Supplementary Figure 5).
STATIN THERAPY AFTER REVASCULARISATION
Statin therapy was applied in 1,346 (74.8%), 1,421 (81.8%), and 1,232 (83.9%) patients at enrolment, discharge and five years after the procedure, respectively (Supplementary Table 4). Non-statin treatment after revascularisation was associated with high mortality, compared to statin treatment (39.1% vs 22.2%, adjusted HR [95% CI]: 1.68 [1.26-2.25], Figure 4A). The beneficial effect of statins was similar in patients with high (≥2 mg/L) or low (<2 mg/L) CRP prior to the procedure (Figure 4B). Regardless of preprocedural values of lipid markers, the mortality rates at 10 years in patients treated with statins after discharge were consistently low (between 21.4% and 23.2%), compared to patients without statins (between 28.8 and 50.9%, Figure 4C-Figure 4E).
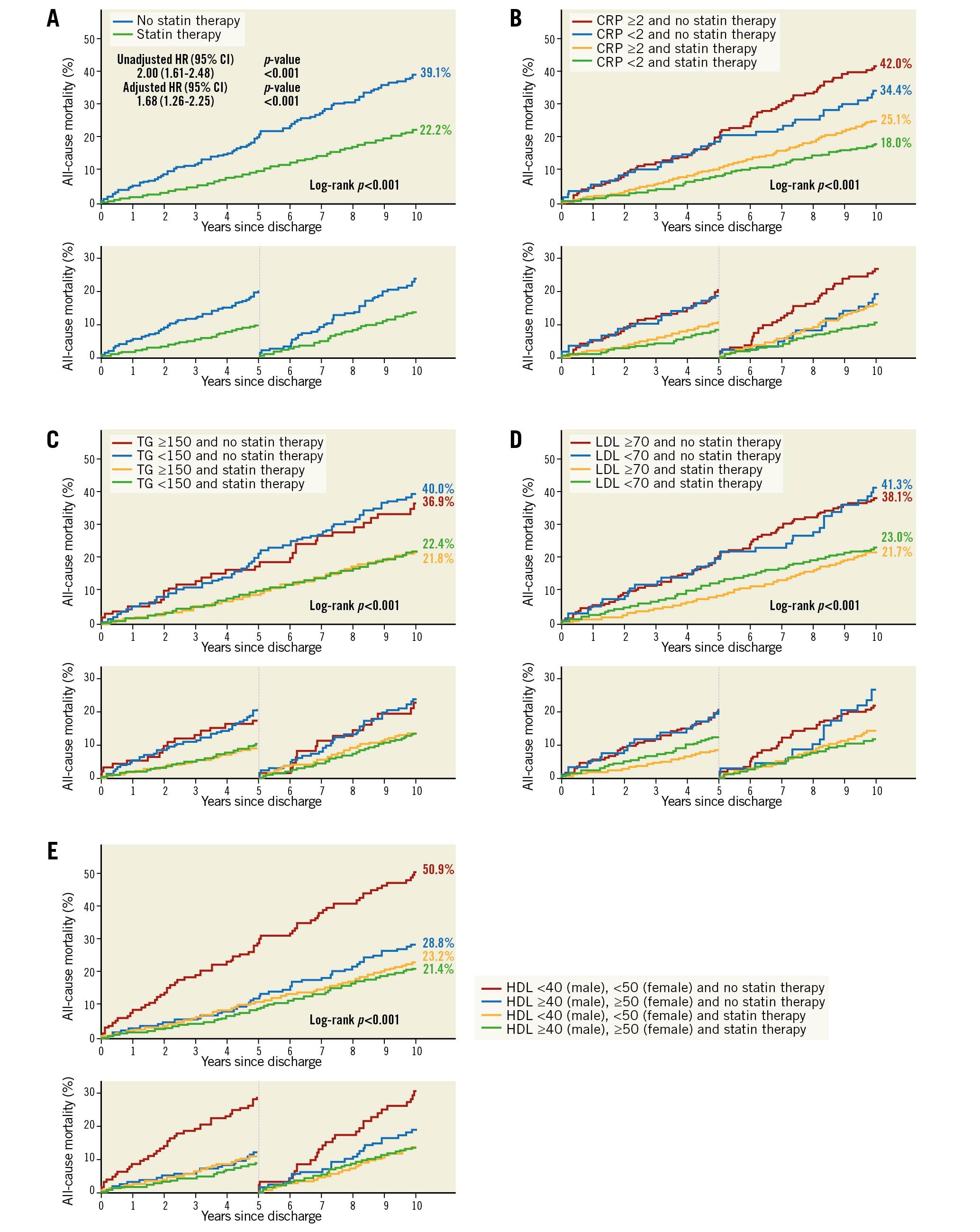
Figure 4. Time-dependent Kaplan-Meier curves for all-cause mortality at 10 years according to the status of statin therapy. Kaplan-Meier curves up to 10 years (upper panel), and in the landmark period of 0 to 5 years and 5 to 10 years (lower panel) are shown. A) Mortality rates according to the status of statin therapy. B-E) Mortality rates according to the status of statin therapy, and (B) CRP, (C) TG, (D) LDL-C, and (E) HDL-C.
THE PREDICTIVITY OF THE SYNTAX SCORE II 2020
As new potential biological markers for the updated SYNTAX score II 2020, CRP, haemoglobin, and HbA1c were incorporated into the process to establish prognostic index, in addition to the variables applied to the original SYNTAX score II 2020 (updated model 1, Supplementary Table 5A). In a second model, the prognostic index was established with CRP alone (updated model 2, Supplementary Table 5B). The calibration plots were similar between the original SYNTAX score II 2020, the updated SYNTAX score II 2020 models 1 and 2. The updated models only slightly increased the c-indexes in the PCI and CABG arms, compared to the original model (Supplementary Figure 6).
Discussion
Very long-term follow-up (10 years) data after PCI and CABG are limited, and the non-biased interaction between PCI and CABG in terms of the association between preprocedural biomarkers and 10-year mortality is only available from the randomised trials. The main findings of the present study are as follows (Central illustration):
(i) High preprocedural CRP, low haemoglobin, and CKD (CrCl <60 ml/min) were associated with 10-year mortality.
(ii) Preprocedural HbA1c was associated with 10-year mortality in the overall population. In patients without medically treated diabetes at enrolment, a level of HbA1c ≥6% (42 mmol/mol) resulted in a worse outcome, compared to patients with HbA1c <6% (42 mmol/mol). However, in diabetes patients, HbA1c did not predict the 10-year all-cause mortality.
(iii) There was no significant interaction observed between PCI and CABG in terms of the association between preprocedural biomarkers and 10-year mortality, suggesting that physicians are not mandated to consider the values of preprocedural biomarkers in decision making between PCI and CABG.
(iv) Statin therapy was associated with decreased risk of mortality regardless of the preprocedural lipid profile and level of CRP. Conversely, the non-use of statins was a factor of a worse prognosis.
(v) Implementation of biological markers into the SYNTAX score II 2020 did not significantly improve predictivity.
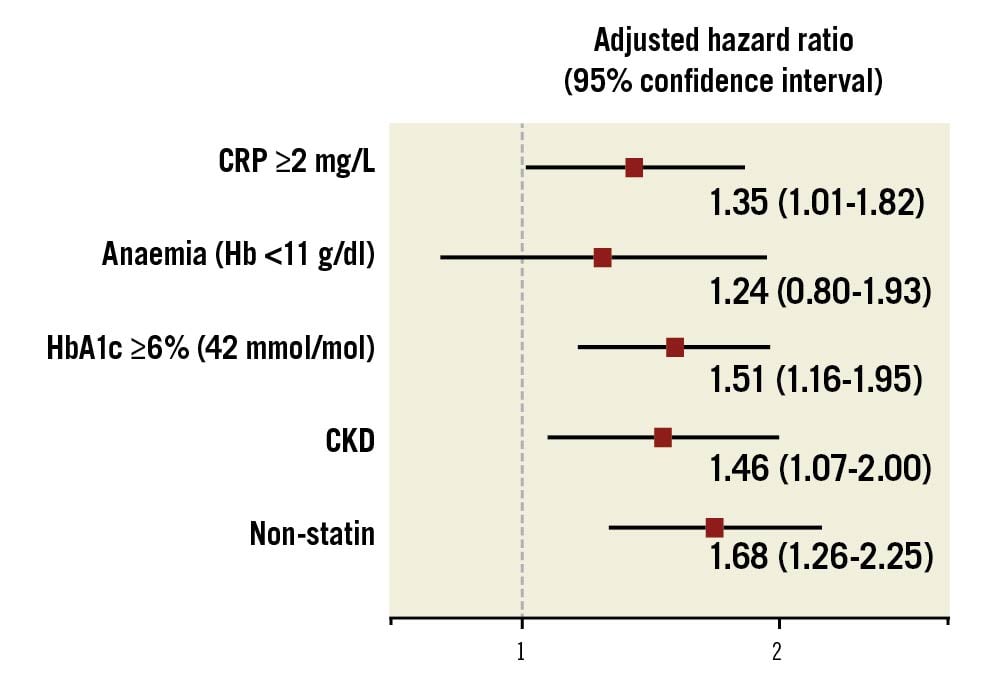
Central illustration. Impact of biological markers and statin on 10-year mortality. CKD: chronic kidney disease; CRP: C-reactive protein; Hb: haemoglobin
CRP
Inflammation, presented as elevated CRP or white blood cell level, was associated with worse angiographic and clinical outcomes after PCI or CABG419. In the EXCEL study, elevated baseline CRP was associated with an increased risk of three-year death, MI, or stroke after LMCAD revascularisation regardless of the revascularisation mode, and the impact of CRP for three-year death, MI, or stroke was not different in patients treated with PCI or CABG (p for interaction=0.75)5. The impact of CRP on all-cause mortality is effectively mitigated by using statins (Figure 4B) but is apparently sustained up to 10 years in the SYNTAXES study (Figure 2A, Figure 3A). The Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Patients With Elevated Risk (FOURIER) and the Studies of PCSK9 Inhibition and the Reduction of Vascular Events (SPIRE) trials demonstrated that CRP remains a strong predictor of future cardiovascular risk, even after treatment with both high-intensity statins and PCSK9 inhibitors2021. Given that CRP is an independent predictor of mortality, though statins are a powerful lipid-lowering agent with anti-inflammatory effects, “residual inflammatory risk” could be the target for risk reduction, as demonstrated in the Canakinumab Antiinflammatory Thrombosis Outcomes Study (CANTOS) trial22.
HAEMOGLOBIN
Although baseline anaemia defined by WHO criteria did not increase major adverse cardiac and cerebrovascular events (MACCE), non-cardiac death, MI, or stroke in the EXCEL trial, it was associated with five-year all-cause mortality (20.4% vs 8.5%; adjusted odds ratio [OR]: 2.00; 95% CI: 1.39 to 2.88; p=0.0002) mainly due to an increase in cardiac death (12.6% vs 4.1%; adjusted OR: 2.26; 95% CI: 1.41 to 3.64; p=0.0007)3. No significant interaction occurred between anaemia status and PCI vs CABG for five-year all-cause mortality (p for interaction=0.34). In the SYNTAXES study, haemoglobin treated as a continuous variable was associated with 10-year mortality, even though moderate or severe anaemia treated as a categorical variable was not associated with mortality. In terms of revascularisation strategy, haemoglobin treated as a continuous variable was significantly associated with 10-year mortality after CABG (adjusted HR [95% CI]: 1.16 [1.04-1.30]), but not after PCI (adjusted HR [95% CI]: 1.06 [0.95-1.19]). Haemoglobin treated as a continuous variable tended to have a greater impact on mortality after CABG, compared to PCI, but the interaction between PCI and CABG was not significant (p for interaction=0.058).
HbA1c
In the Atherosclerosis Risk in Communities (ARIC) study with 11,092 non-diabetic participants, HbA1c was a marker of 15-year cardiovascular events and all-cause death. The J-shaped relation between HbA1c value and all-cause death was observed and HbA1c less than 5.0% (31 mmol/mol) could also increase all-cause mortality risk23. A similar association was found between continuous HbA1c measures and all-cause mortality after 11.6 years of follow-up among participants without known diabetes in a nationwide German cohort24. In a meta-analysis, the optimal HbA1c associated with the lowest all-cause and cardiovascular mortality ranged from 5.0% (31 mmol/mol) and 6.0% (42 mmol/mol) in the non-diabetic population and 6.0% (42 mmol/mol) and 8.0% (64 mmol/mol) in the diabetic population15. In the SYNTAXES study, the same association was observed (Supplementary Figure 4B): HbA1c less than 5% (31 mmol/mol) was observed only in 28 patients, and patients with HbA1c ≥6% (42 mmol/mol) resulted in a worse outcome compared to patients with HbA1c <6% (42 mmol/mol) without medically treated diabetes. In patients with medically treated diabetes, preprocedural HbA1c did not predict the mortality.
However, more than half of patients with medically treated diabetes at enrolment interrupted their medication for diabetes in their first five years of follow-up and these patients had a higher risk of all-cause mortality. In patients with diabetes, adherence to pharmacological therapy after revascularisation is more predictive for all-cause mortality than the preprocedural level of HbA1c in itself.
PREPROCEDURAL CREATININE LEVEL
CrCl previously incorporated into the SYNTAX score II published in 2013 was maintained as a predictor of mortality in the SYNTAX score II 202012. The FREEDOM score for five-year MACCE also uses CrCl and predicts five-year MACCE rates after PCI or CABG in patients with diabetes mellitus and 3VD without LMCAD25.
It has not been fully evaluated whether PCI or CABG should be preferred in patients with CKD (CrCl <60 ml/min), whereas CKD is strongly associated with increased cardiovascular mortality. In the EXCEL trial, there were no significant differences between PCI and CABG in terms of death, stroke, or MI at three years in patients with and without CKD17. In the FREEDOM trial which exclusively enrolled patients with diabetes and multivessel disease, the treatment effects between PCI and CABG on death, stroke and MACCE at five years were comparable in patients with and without CKD, although rates of MI and repeat revascularisation were significantly lower with CABG than with PCI irrespective of baseline renal status26. In terms of long-term follow-up, the randomised MASS II trial comparing PCI with bare metal stents, CABG, and medical therapy investigated 10-year results according to kidney function. However, the number of CKD patients in the PCI and CABG arms was 49 and 47, an insufficient number for the assessment of treatment effects between PCI and CABG27.
In the present study, CKD was associated with 10-year all-cause mortality but there was no significant interaction for the impact of CKD on mortality between PCI and CABG (p for interaction=0.356, Supplementary Figure 2D), an observation which is consistent with the three-year results of the EXCEL trial and the five-year results of the FREEDOM trial.
LIPID MARKERS
In the SYNTAXES study, preprocedural TG, LDL-C, and HDL-C were not associated with 10-year all-cause mortality. It has been reported that statin therapy could reduce cardiac events and low LDL-C after revascularisation and is associated with improved long-term cardiac outcomes28. Although the follow-up values of lipid markers were not collected in the SYNTAXES study, statin therapy documented up to five years after revascularisation reduced the 10-year mortality regardless of preprocedural TG, LDL-C, and HDL-C, which means that statin therapy could be a potent “effect modifier” after revascularisation and when treated, preprocedural lipid abnormalities are no longer predictive markers for long-term mortality. At enrolment in the trial, only 70 patients (43.5%) with preprocedural LDL ≥140 mg/dl were treated with statin but after procedure, 105 patients (66.0%) and 114 patients (78.6%) were under statin treatment at discharge and five years, respectively (Supplementary Table 6). The biphasic appearance of the cubic spline curve for adjusted HR of LDL-C (Figure 2F) may reflect a risk reduction by starting statin therapy after procedure mainly in patients with LDL ≥140 mg/dl.
FUTURE RISK SCORE AND IMPACT OF PHARMACOLOGICAL TREATMENT
Incorporation of CRP, haemoglobin, and HbA1c into the SYNTAX score II 2020 did not significantly improve its predictiveness. Cox regression was used to develop a clinical prognostic index for predicting 10-year all-cause death, although biological markers did not demonstrate perfect linear relationships with outcomes. Other methods, such as machine learning algorithms, may provide better predictivity, and should be investigated in the future29.
HbA1c was associated with all-cause mortality, an effect mainly observed in patients without medically treated diabetes and fully obviated in patients with good adherence to their pharmacological treatment, for at least up to five years. In a similar fashion, all-cause mortality associated with preprocedural lipidic markers could be effectively mitigated by a medical treatment such as a statin.
Limitations
First, as this is a post hoc analysis, all the presented findings must be interpreted as hypothesis-generating due to the inherent limitations of post hoc analysis, including multiple testing30. Second, the first-generation TAXUS DES and a conventional PCI strategy of implantation were used in the SYNTAX trial. The TAXUS stent is no longer commercially available and newer-generation DES have improved clinical outcomes. It is, however, unavoidable that the findings from long-term follow-up data are based on partially outdated technology while the evidence for contemporary technology can be derived only from short-term follow-up studies. Third, the endpoint in the SYNTAXES study was only all-cause death and adherence to pharmacological treatment was only available up to five years. Their continuation beyond that period of observation relies on a hypothetical assumption. In addition, pharmacological treatment was not randomised in the SYNTAXES study. Fourth, the SYNTAXES study provides the first randomised data that were meticulously collected and achieved a high follow-up rate of 93.8% for 10-year vital status, but the completeness of the follow-ups differed between countries. Fifth, biological markers were not collected during the follow-up and decision making between PCI and CABG is theoretically only based on the baseline biological markers. Thus, we could not investigate the association and interaction between biological markers, implementation of optimal pharmacological treatment and all-cause mortality after revascularisation which could impact on clinical practice. Sixth, lipid particles, such as Lipoprotein(a), and WBC were not collected at baseline, therefore we could not analyse the impact of these biomarkers.
Conclusions
Preprocedural biomarkers, such as CRP, haemoglobin, and HbA1c were associated with long-term mortality post revascularisation, regardless of the revascularisation techniques. In patients with medically treated diabetes, HbA1c was not associated with the 10-year all-cause mortality, emphasising the importance of pharmacological treatment. Similarly, the high risk of cardiovascular events, associated with conventional lipidic biomarkers and inflammatory biological markers such as CRP, seems to be effectively mitigated by the long-term use of statins, whereas the non-use of statins was a factor of a worse prognosis.
Impact on daily practice
Creatinine clearance is an independent determinant of mortality in predictive models of revascularisation outcome for complex coronary artery disease. In addition to creatinine clearance, preprocedural biomarkers, such as CRP, haemoglobin, and HbA1c were associated with long-term mortality post revascularisation.On the other hand, all-cause mortality associated with preprocedural lipidic markers could be effectively mitigated by a medical treatment such as statins, which emphasises the impact of secondary prevention.
Acknowledgements
We thank all research coordinators, cardiothoracic surgeons, and cardiologists at participating hospitals who contributed to the SYNTAXES study.
Funding
The SYNTAX Extended Survival study, which extended the follow-up up to 10 years, was supported by the German Foundation of Heart Research (Frankfurt am Main, Germany). The SYNTAX trial, covering the 0-5 year follow-up, was funded by Boston Scientific (Marlborough, MA, USA).
Conflict of interest statement
H. Hara reports a grant for studying overseas from the Japanese Circulation Society and a grant from the Fukada Foundation for Medical Technology. M.C. Morice is CEO and shareholder of CERC, a CRO not involved in this trial, and is a minor shareholder of ELECTRODUCER. A. Kappetein reports working as an employee of Medtronic, outside the submitted work. P.W. Serruys reports personal fees from Biosensors, Micel Technologies, Sinomedical Sciences Technology, Philips/Volcano, Xeltis, HeartFlow, and SMT outside the submitted work. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

