
The first criteria for MI definition, used in collaborative projects in the 1970s, were based on the World Health Organization (WHO) European acute MI registry which was later revised in a document published by the WHO together with the International Society and Federation of Cardiology1,2. This definition was based on clinical history, electrocardiographic (ECG) changes (ST-elevation and Q-wave), serum biomarkers and post-mortem findings. In a revised version of the definition, the Minnesota coding was used to standardise ECG criteria3.
This led to a classification of Q-wave and non-Q-wave MIs, used in research protocols and clinical trials. In cases of equivocal ECG changes (i.e., non-Q-wave MIs), release of creatine kinase (CK) was used for diagnosis. This enzyme can be released in the circulation by different tissues in the organism. To avoid a mistake related to the non-cardiac CK, the specific isoenzyme (CK-MB) was required and the classic definition of non-Q-wave MI used in stent trials became the elevation of CK ≥2x the upper limit of normal (ULN) with the presence of elevated isoenzyme (CK-MB) levels4,5. This definition remained widely used in trials for many years until CK-MB elevation took over and became the criterion, used in a second wave of trials6-10.
CK-MB values of 3x or 5x the ULN were used because they were shown to be associated with a prognostic aspect in terms of long-term mortality. In the ARTS trials we even observed that, for patients undergoing coronary artery bypass surgery (CABG), CK-MB levels ≥3x ULN had a prognostic value. In this case, the myocardial injury was probably related to poor quality of cardioplegia, rather than side branch occlusion, as in PCI.
At this point, a digression must be made. At the time of the introduction of glycoprotein (GP) IIb/IIIa receptor antagonists, CK-MB was (mis)used as a surrogate for the beneficial effect of the drug on the platelet function. It took us some time to realise that the surrogate was not, per se, the demonstration that GP IIb/IIIa use would reduce mortality11.
The next step was the introduction of cardiac troponin thanks to the work of Hugo Katus and Christian Hamm, who quite early on published, in the New England Journal of Medicine, the use of troponin as a more specific, sensitive and earlier marker of myocardial injury12.
In the early days of coronary stenting, the big corporations were anxious to have unified criteria for study outcomes in order to be able to pool their data and to compare their devices. This is one of the main reasons why the WHO MI definition was maintained in device industry-sponsored clinical trials. At that time, several discussions took place between investigators and the industry because new biomarkers could not be introduced due to reasons of poolability and comparability of data.
On the clinical side, cardiologists and intensive care physicians working in acute coronary care units were very much attracted by the accuracy of troponin to avoid misdiagnosis of non-STEMI. Then, a group of physicians led by Allan Jaffe and Harvey White provided an update of the previous consensus in MI definition13, in which they recommended troponin as the preferred biomarker to be used in what became the so-called universal definition14. That document evolved with the idea of a classification that would cover the entire spectrum of myocardial infarction. In addition to the classic spontaneous MI related to coronary atheroma plaque rupture, they described oxygen supply/demand mismatch, periprocedural injury following PCI and CABG, and sudden death15.
Nevertheless, somewhere in that process, a kind of friction emerged between the world of the interventional cardiologists and that of the acute care physicians. An example of this can be found at the time of the Academic Research Consortium (ARC) definition, in which a consensus of several researchers from different societies of interventional cardiology decided to use CK-MB 3x ULN as the criterion for non-Q-wave MI16.
That document, defining criteria for stent thrombosis, target lesion revascularisation and periprocedural MI, was submitted to the Journal of the American College of Cardiology (JACC) and was rejected by the journal, probably because the ACC, AHA and other societies were not properly represented in the ARC study group. The document was finally published in Circulation. At the time of the final review and rebuttal of the manuscript, reviewers requested that troponin be included together with CK-MB. Technically, a mistake was made in which CK-MB 3x ULN was considered equivalent to troponin 3x ULN. This was the origin of a long misunderstanding and misinterpretation of clinical trial results.
Later, a new definition system was created during the adjudication of events in the RESOLUTE All-Comers trial, a Medtronic-sponsored study17. In that trial, it was not until 70% of the patients were recruited that it became evident that CK and CK-MB, the study criteria for MI, would not be available in a sizeable proportion of patients because of biomarker collection compliance issues. Thus, in an emergency, a working group was created to review all biomarkers collected in the trial and to try to come up with a hierarchical algorithm to define the MI criteria for the patients lacking CK and CK-MB data.
The new definition was named “WHO extended” and was published by Pascal Vranckx et al in a simple four-page document in EuroIntervention18. This was carried out basically in order to facilitate the adjudication process in the RESOLUTE All-Comers trial.
Following that experience, in the next trial, which was a study on bioresorbable scaffolds (the ABSORB II trial), the principal investigator requested something quite new and unique from the sponsor (Abbott Vascular): a core laboratory for cardiac biomarkers and high compliance with multiple biomarker collections (CK, CK-MB, troponin) at multiple time points pre and post procedure, especially in patients with post-procedure elevated enzymes (Figure 1)19. The striking feature of this trial was that the compliance for biomarker collection was 97.8%.
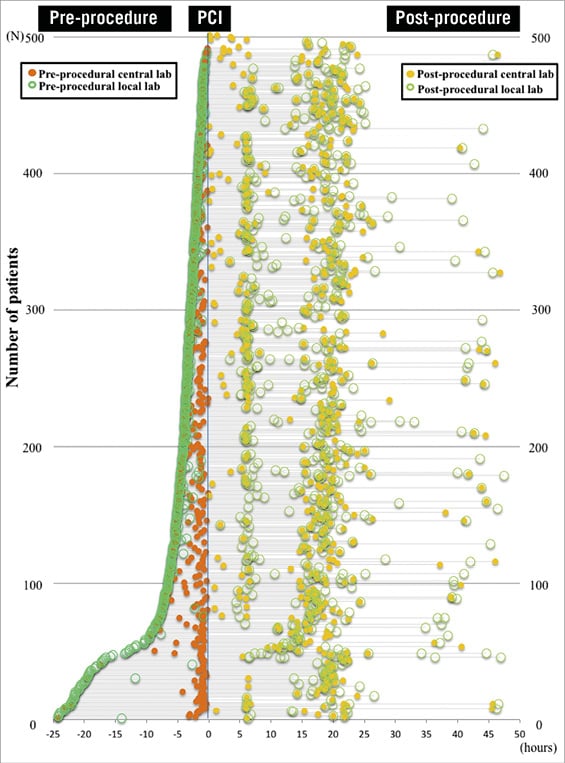
Figure 1. Time points and availability for the assessment of cardiac biomarkers pre- and post-procedure in the ABSORB II trial. A total of 920 blood time points for the assessment of cardiac biomarkers (CB) were available with 458 central and 462 local biomarker data within 24 hrs before the index procedure. At least one of the three CB was available in 486 patients (97.0%) within 6 hrs and in 495 patients (98.8%) within 24 hrs before the index procedure. At least one of the three CB was available in 490 patients (97.8%) within 48 hrs after the index procedure. In the serial sample analysis, 1,446 blood time points for the assessment of CB were available with 572 central and 874 local biomarker data. Reproduced from Ishibashi Y, et al. JACC Cardiovasc Interv. 2015;8:1053-63. With permission from Elsevier.
The lesson learned with the ABSORB II experience was that, in a trial of simple coronary lesions (58% of type A/B1 lesions), using the enzymatic criteria of the third universal definition alone (troponin ≥5x URL) periprocedural myocardial infarction (PMI) was present in 29.7% (ABSORB BVS arm). Using the protocol definition of CK 2x ULN + CK-MB, it was present in 3.9% (ABSORB BVS arm), while taking CK-MB 10x ULN, as proposed in the SCAI definition, it was present only in 0.6% (both arms)19. This demonstrates, rather disturbingly, the large heterogeneity in enzyme criteria and MI definitions.
These issues have a major impact in clinical trials, especially regarding power and sample size calculations. Nowadays, composite event rates in trials are usually around 6% to 8.5%, with non-inferiority margins of 4.5%. In these composite rates, mortality accounts for 0.5%, target lesion revascularisation for 4 to 5% and MI (PMI and spontaneous) for 2 to 3%. To put these numbers into perspective, we note that, in the ABSORB II trial, where troponin leak was 27.9% (troponin ≥5x URL) as mentioned above, after careful review of all cases for side branch occlusion, symptoms, ECG adjudication and wall motion abnormalities, the final PMI rate according to the third universal definition was still 10.6% (ABSORB BVS arm) and 14.2% (XIENCE stent arm).
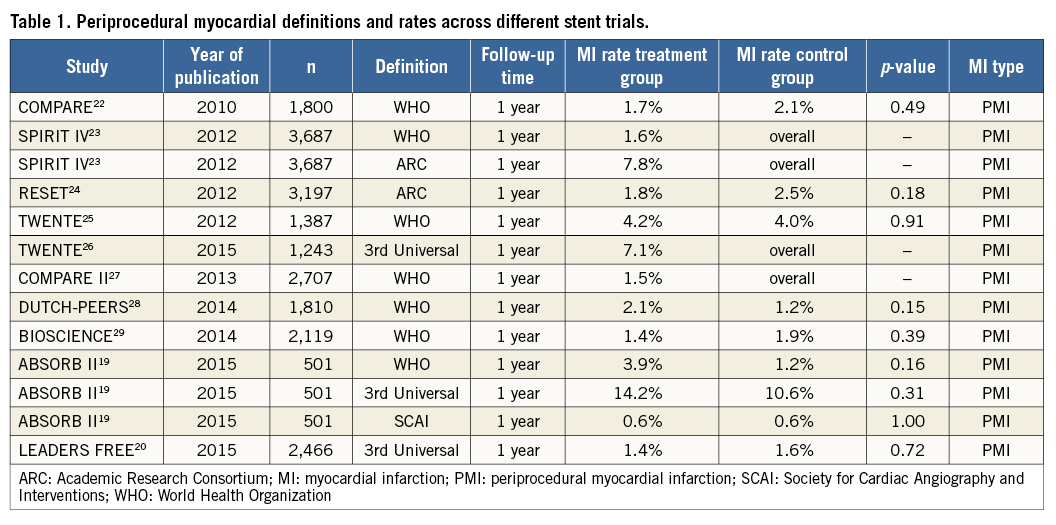
Thus, it is shocking that the periprocedural MI rate alone is higher than the total composite endpoint rate. For trialists, this is a major inconsistency that does not seem to shock the readers of major clinical journals too much. This is clearly exemplified in the LEADERS FREE trial which, including a complex high-risk population, reported a PMI rate of only 1.4% using the third universal definition20,21. This demonstrates that trialists have trivialised the concept of periprocedural MI and that it should be either abandoned or characterised by something robust that is universally accepted and used (Table 1). To claim to have used a definition while failing to comply with an acceptable sampling rate simply indicates that we, as trialists, are not doing a good job. The reliability of global event rates of trials is at stake if PMI is not clearly defined and compliance with data collection is insufficient.
Conflict of interest statement
P.W. Serruys is a consultant for Abbott Laboratories, Astra Zeneca Phamaceuticals, Biotronik, Medtronic, Volcano Europe BVBA, St. Jude Medical, Stentys France, Svelte Medical Systems Inc, Sino Medical Sciences Technology Inc. R. Cavalcante has no conflicts of interest to declare.

