Background
In 2006, in an effort to standardise event reporting between clinical trials, the Academic Research Consortium (ARC), proposed standardised consensus definitions for clinical endpoints in coronary stent investigations in stable patients with de novo lesions and made them available to any and all interested parties via peer reviewed publication.1,2 After careful deliberation, the ARC adopted the joint ESC/ACCF/AHA/WHF (European Society of Cardiology, American College of Cardiology, American Heart Association and World Heart Foundation) task force 2007 universal definition of myocardial infarction for consistent application across investigational studies.3
The term myocardial infarction (MI) refers to myocardial necrosis in a clinical scenario consistent with acute myocardial ischaemia.4 The advent of more sensitive and specific cardiac, troponin I and T, assays to detect myocardial necrosis impacted the 2007 redefinition of MI and may have critical consequences for the analysis and reporting of safety and efficacy data in stent trials. The global task force recommends the implementation of a set of criteria to define myocardial necrosis based on troponin and/or creatinine kinase MB CKMB-mass, but notes the preference of troponin in all cases, provided normal or decreasing troponin levels before the index procedure4. Opposed to this recommendation, the ARC maintained CKMB-mass as the biomarker of choice for the diagnosis of periprocedural MI.1
Concerns were voiced over whether troponin may prove over-sensitive as biomarker of myocardial necrosis in coronary stent trials, especially in all-comers “real world” patient populations. In addition, legacy data may exist that did not use troponin values, making it difficult to reconcile previous trials with current/ongoing investigations, new standards need to be set to allow comparisons between stent trials. Moreover, with the apparent “demise”5 of Creatine Kinase-MB (CKMB) some centres removed CK-MB from its cardiac biomarker panel, yet others did not make the transition to troponin only. As coronary stent trials evolve to include “real world” populations in both pre- and post-regulatory approval settings, an abrupt transition to troponin as the sole biomarker of myocardial necrosis poses two critical challenges: 1) The majority of historical coronary stent trial data does not utilise troponin values to define myocardial infarction, thus, making it difficult to compare previous trials with current/ongoing investigations; and 2) troponin as a biomarker of myocardial necrosis may prove overly sensitive and non-linear, thereby, elevating the overall rates of myocardial infarctions and diluting potential clinical differences based on size of injury.
The representatives of three international academic research organisations (AROs) considered these challenges in their efforts to harmonise clinical event adjudications for the Medtronic (Medtronic, Minneapolis, MN, USA) RESOLUTE clinical program.
The Medtronic RESOLUTE clinical program is a global program that will evaluate the performance of the Endeavor® Resolute Zotarolimus-Eluting Coronary Stent System versus an active control and against historical controls from the ENDEAVOR clinical program (Figure 1). The RESOLUTE clinical program will report endpoints according to the 2007 ARC universal definition of MI and the historical definitions of MI utilised in pivotal DES studies. The dual reporting framework will facilitate a transition to the 2007 ARC universal definitions while also allowing a “bridge” back to the historical data from previous coronary stent trials. This technical report aims to create transparency and to provide insights to the MI adjudication process based on the CK(MB) based historical definitions.
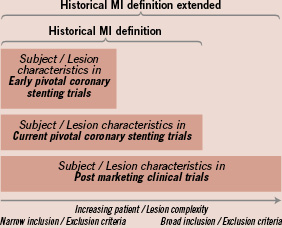
Figure 1. Implementation of the (extended) MI definitions across the Endeavor Stent Trial programme to allow for between trials meaningful comparison.
The historical MI definitions used in early DES stent trials extended to accommodate real world populations
In an effort to adequately define efficacy, early DES stent trials evaluated highly selected subject cohorts undergoing planned percutaneous coronary interventions (PCI); the historical MI definitions facilitated clinical event adjudication in this setting. The number of clinical trials in a post marketing, “real world” context has increased; at the same time, pre-marketing clinical trials have extended inclusion/exclusion criteria to include broad, unselected patient populations. While the historical MI definitions are appropriate for adjudicating a significant portion of events in both highly selected subject cohorts and broad, unselected subject cohorts, we aimed to extend the definitions to account for both cohorts in order to completely link historical and current data sets.
Table 1 lists the different components of the definition considering the initial clinical setting. In a clinical setting consistent with acute myocardial ischaemia presentation, criteria are presented for defining MI that include a rise and/or fall in biomarkers of myocardial damage (BMD) together with symptoms of ischaemia, and/or appropriate ECG changes. MI during a trial of intracoronary devices may occur during the immediate periprocedural period related to the index procedure or long after the procedure as result of spontaneous MI or late complications of the study device or subsequent revascularisation procedures. Due to differences in expected rise and fall of cardiac biomarkers, we have defined the periprocedural period as the first 48 hours after PCI and first 72 hours after coronary bypass grafting (CABG).

The minimal clinical data requirements for MI event adjudication include a 12-lead electrocardiogram recorded before the procedure and at least one repeat recording within 24 hours or at discharge, whichever comes first. A 12-lead ECG should be repeated in case of suspected acute ischaemia. Biomarkers of myocardial damage (BMD) should be assessed at baseline, 6-8 hours later with follow-up every 6-8 hours if elevated until peak noted. A systematic analysis of CPK, CK-MB band and/or troponin curves is required to detect reliably unreported MI’s, but it is unlikely that these data would be consistently available. A hierarchical approach is proposed to the clinical event committees (CEC) for adjudication when one or more biomarkers is missing. In this process measurement of mass concentration of CKMB is preferred over CKMB activity.
Periprocedural MI following PCI may occur in stable patients, with baseline biomarker levels below the upper limit of normal, or in the setting of an evolving (infarction extension) or recent (re-infarction) MI. Since historical clinical trials generally excluded these patients, it is necessary to modify the historical definitions to allow for adjudication of these events in the case of evolving MI a new event is triggered by clinical symptoms or ECG changes indicative of new myocardial ischaemia according to the Minnesota Code Classification.6 Different scenarios may be considered taking into account the pre-PCI biomarker status (Figure 1). In case biomarkers have not yet peaked (Scenario A, Figure 2); myocardial (re-)infarction extension in the setting of PCI requires new signs or clinical symptoms of myocardial ischaemia accompanied by an additional 50% elevation of BMD (preferably CK using the historical definitions) within 24 hours after the index procedure and above a pre-specified threshold. The 24 hours time frame links the biomarker data to the new clinical signs or symptoms. After the biomarker has peaked (Scenario B-C, Figure 2) any significant rise starting within the 48 hours following PCI will define a (re-)MI (extension). For patients referred to CABG different thresholds for biomarkers and time-frames are proposed. The committee noted that clinical symptoms should not be required in patients that are intubated, sedated or under the intra-operative or postoperative effects of anaesthesia. Early staged procedures (Table 2: definition) in the presence of elevated biomarkers at the time of the second intervention will be evaluated in this latter category.
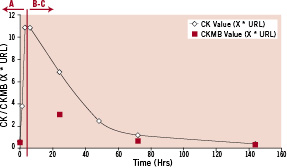
Figure 2. Expected pattern of (CK)MB release in the context of an ST-segment elevation MI. URL: upper reference limit.

Aligned with the historical definition, spontaneous MI required either clinical symptoms of MI and the development of pathological Q waves in at least two contiguous leads with a confirming biomarker (preferably CKMB) or, in the absence of pathological Q waves, an elevation in CK to greater than twice the upper limit of normal in the presence of a confirming biomarker (preferably CKMB). With sudden, unexpected cardiac death, involving cardiac arrest, especially when accompanied by clinical signs or symptoms of myocardial ischaemia, the CEC may adjudicate MI considering the clinical scenario including pathological findings, new wall motion abnormalities on non-invasive imaging. However, of note, silent MI was not considered per the historical definition.
The 2007 ARC universal definition of myocardial infarction specification
The 2007 ARC universal definition of myocardial infarction has been detailed in a previous publication.1 When considering the 2007 ARC universal definition of myocardial re-infarction following PCI (type 4a) multiple elements need to be highlighted. Re-infarction in the setting of primary PCI can only be assessed if troponin (or CKMB in the absence of troponin) is falling on serial measurements with 3-6 hours difference after the index event (Figure 2, scenario B-C) and requires new clinical signs or symptoms of MI. Program checks of biomarker data will only consider troponin values that are falling or have a rise <10%, correcting for the coefficient of variation of troponin at the level involved with reinfarction A 20% rise is considered significant, i.e., over that expected from analytical variability itself, however, we this value must exceed the appropriate threshold according to the timing of the event (post-PCI, post-CABG, or spontaneous).
Conclusion
The expanded ability to detect myocardial injury using very sensitive and specific biomarker assays has been a major factor in the 2007 ARC universal definition of MI. The implementation of “better-performance” assays should be welcomed; moreover, the interventional cardiology community should seek to acquire additional experience in clinical investigations evaluating the safety and efficacy of new coronary stent technology. One should bear in mind that the sole implementation of troponin to define MI post-PCI has high (possibly too high) sensitivity and will inflate the number of periprocedural MI and by consequence major cardiac events. We have provided a framework to allow for inter trial standardisation and linkage to historical control data based mostly on total CK measurements. Importantly, criteria have been added to the historical definitions to allow their implementation in contemporary all-comers stent trials including emergency PCI patients.

