
Abstract
Aims: To evaluate the prognostic implications of baseline cardiac troponin (cTn) values in the normal range in stable coronary artery disease (CAD) patients successfully treated with percutaneous coronary intervention (PCI).
Methods and results: We investigated the correlation between pre-procedural cTnI levels and major clinical adverse events at three years of follow-up in 1,063 consecutive stable CAD patients with normal baseline cTnI levels, successfully treated with PCI. Patients with pre-procedural cTnI levels in the upper tertile showed an increased long-term risk of overall death (HR 3.17, 95% CI: 1.62 to 6.21; p=0.0001), cardiac death (HR 5.09, 95% CI: 2.30 to 11.25; p=0.002), myocardial infarction (MI) (HR 2.34, 95% CI: 1.45 to 3.76; p=0.003) and target vessel failure (TVF) (HR 1.91, 95% CI: 1.28 to 2.84; p=0.006). Pre-procedural cTnI levels remained significantly correlated after adjustment for clinical and angiographic findings. Analysis of pre-PCI values eliminated any association of post-PCI values with prognosis.
Conclusions: In stable CAD patients successfully treated with PCI, pre-procedural cTnI levels, in the upper limits of the normal range, are associated with hard cardiac endpoints.
Introduction
Increased baseline cardiac troponin (cTn) levels before percutaneous coronary intervention (PCI) are associated with adverse outcomes1, probably because they are a marker of the extent and complexity of coronary artery disease (CAD)2 and plaque instability3,4.
Studies have shown that cTn levels near but below the 99th % URL have prognostic importance in patients with acute coronary syndromes5,6, left ventricular hypertrophy7, cor pulmonale7, stable coronary artery disease7-9, and even in subjects without overt cardiac disease10. Thus, an arbitrary cut-off such as the 99th % URL is too high to exclude subclinical disease11. Accordingly, we probed whether baseline cTn below the 99th % URL would augment the importance of baseline cTn values. We measured cTn with a sensitive third-generation assay in patients with clinically stable CAD undergoing uncomplicated elective PCI.
Methods
PATIENT POPULATION
Patients with stable angina or silent ischaemia scheduled for elective procedures and angiographically successful PCI with stent deployment (<10% residual stenosis based on visual estimation, TIMI 3 final flow, and no angiographically detectable side branch loss, dissection or distal embolisation) were included.
Those with acute coronary syndromes within three months before PCI, pre-procedural cTnI above the 99th % URL, haemodynamic instability, staged PCI, rotational or directional atherectomy, saphenous bypass graft intervention, and/or severe systemic illness or malignancy were excluded.
Patients with transient slow or no-reflow were included, while those with persistent slow or no-reflow and those with angiographically detectable residual dissections, branch losses or distal emboli were excluded.
Demographic and clinical features and major adverse events (death, myocardial infarction [MI], repeated coronary revascularisation) were collected.
PERCUTANEOUS CORONARY INTERVENTION
PCI was performed according to the operator’s preferences. Four to six hours before PCI, patients not on aspirin and clopidogrel received: 1) aspirin 300 mg and 100 mg daily dose thereafter; 2) clopidogrel 600 mg followed by 75 mg daily for at least one month following BMS and 12 months following DES. Unfractionated heparin, bivalirudin, and/or intravenous glycoprotein IIb/IIIa inhibitor use were at the discretion of the attending physician.
PROTOCOL FOR SAMPLE ACQUISITION
Samples were obtained before PCI and 24 hours after. They were centrifuged and tested for cTnI using the ultrasensitive third-generation assay (Advia Centaur TnI-Ultra assay; Bayer, Pittsburgh, PA, USA). The limit of detection for this assay is 0.006 ng/dL, total imprecision of 10% occurs at 0.03 ng/dL, and the 99th percentile value is 0.04 ng/dL.
STUDY ENDPOINTS
The main endpoint was overall death. Secondary endpoints included: cardiac death, defined as death caused by coronary heart disease or other diseases of the heart; MI, defined according to the third universal definition12; target vessel failure (TVF), due to restenosis ≥50% with recurrent angina or evidence of myocardial ischaemia; or restenosis ≥70% by angiographic estimation.
Follow-up began 24 hours after PCI and lasted for three years. All secondary endpoints were ascertained by two senior cardiologists (A. Lupi, G.G. Secco). Only the initial event was counted in patients with multiple events.
ETHICAL ISSUES
The study fulfilled local institutional review board guidelines and conformed with the Declaration of Helsinki13.
STATISTICAL ANALYSIS
Normally distributed data are presented as means±standard deviation (SD) for continuous variables, skewed data as medians and interquartiles, and dichotomous variables as proportions. Differences in prevalence were compared with the chi-square test or Fisher’s exact test for dichotomous variables. Continuous variables were compared with the Student’s t-test or Wilcoxon signed rank-sum test.
Univariate correlations between baseline cTnI levels and other variables were assessed by Pearson’s or Spearman’s correlation. Multivariate logistic regression was performed for predictors of baseline cTnI levels in the upper tertile. Covariates tested were male sex, age, hypertension, diabetes, dyslipidaemia, body mass index (BMI) >30 kg/m2 of body surface area (BSA), estimated glomerular filtration rate (eGFR) <30 mL/min, cigarette smoking (active or quit less than two years), previous MI and/or PCI, previous CABG, peripheral artery disease, left ventricular ejection fraction (LVEF), fibrinogen, high sensitivity C-reactive protein (CRP), drug treatment before PCI with aspirin, clopidogrel, statins, beta-blockers or ACE inhibitors/AT2 antagonists, multivessel CAD, left main, type B2/C lesion. Univariate predictors with a p<0.10 were included in multivariate modelling.
Baseline cTnI measurements were divided into tertiles and outcomes were stratified, calculated and plotted with the Kaplan-Meier method. Comparisons were performed with log-rank tests.
Relations between cTnI and endpoints were assessed with Cox proportional hazards models. Univariate associations with endpoints were estimated for all clinical and procedural variables. To test the independence of baseline values, cTnI was entered into a multivariable Cox proportional hazards model which also included univariate predictors of mortality with p≤0.10).
A p-value <0.05 was considered significant. Analyses were performed with SPSS statistical software package version 16.0 (SPSS Inc., Chicago, IL, USA).
Results
BASELINE CHARACTERISTICS
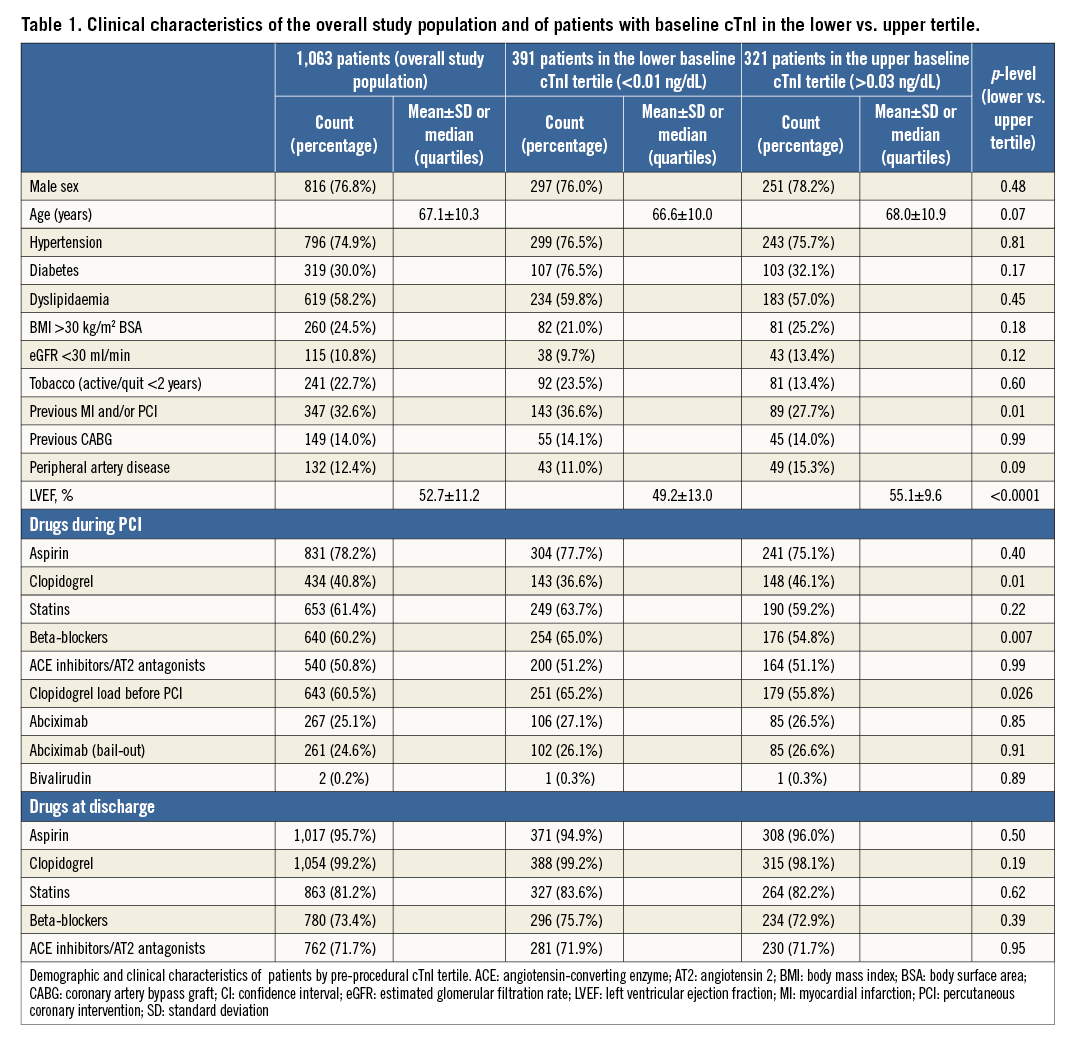
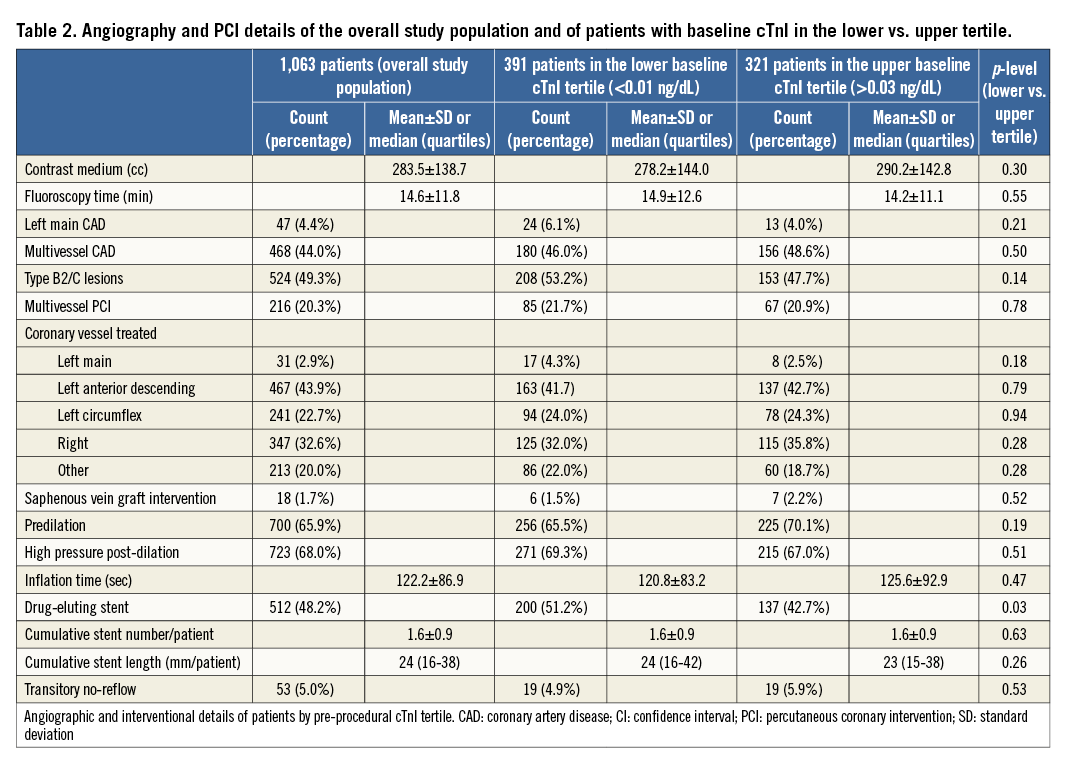
From January 2007 to January 2010, 1,249 consecutive patients underwent elective PCI; 1,063 (85.1%) fulfilled inclusion criteria. Clinical features are shown in Table 1 and details of their procedures in Table 2.
BASELINE cTnI LEVELS
Baseline cTnI was below the URL (0.04 ng/mL) in all patients (median 0.02 ng/dL [0.01-0.03 ng/dL]).
Compared to the lowest tertile, patients in the highest tertile manifested more previous cardiac disease and required more frequent clopidogrel loading. Univariate correlations were present between baseline cTnI and fibrinogen and CRP levels (Table 3); LVEF correlated inversely with baseline cTnI (Table 1).
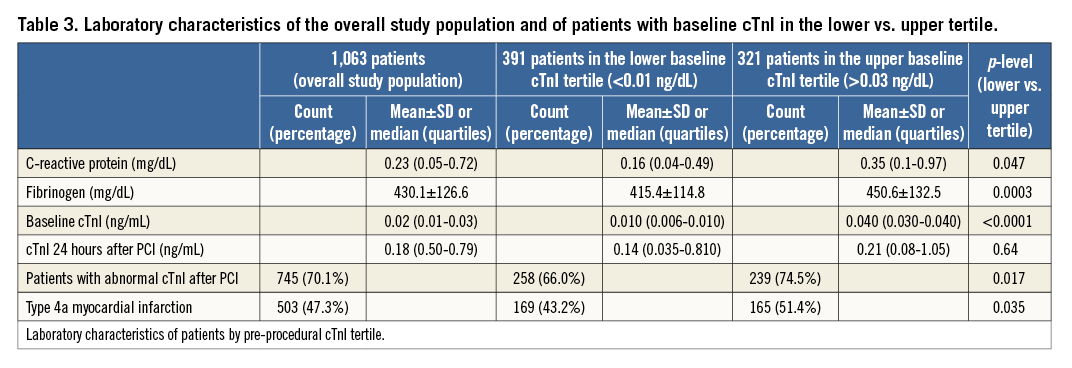
cTnI increased post procedure to a median of 0.18 ng/mL (0.05-0.79 ng/mL, p<0.0001 vs. baseline). Post-procedural cTnI elevations above the URL occurred in 70.1% of patients and met the criteria for a type 4a MI in 47.3% (Table 3).
Angiographic and interventional characteristics were similar across tertiles (Table 2). There was a significant univariate correlation between pre-PCI cTnI and multivessel CAD (rho=0.06, p=0.04) and a weak correlation between baseline and post-procedural cTnI values (rho=0.13, p<0.0001). After PCI, patients in the highest baseline tertile more frequently had abnormal cTnI levels and more type 4a MIs (Table 3).
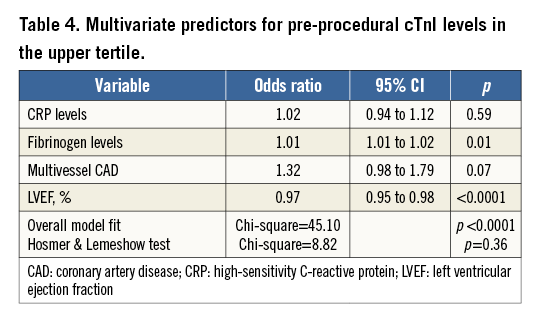
Multivariate analysis demonstrated that only fibrinogen and LVEF were independently associated with pre-procedural cTnI values in the upper tertile (Table 4).
BASELINE cTnI LEVELS AND OUTCOMES
Follow-up (median 2.8 years [1.7 to 3.8 years]) was 100% complete. Mortality occurred in 5.0%, cardiac death in 3.6%, and MI in 9.9%. More (17.9%) underwent symptom-/ischaemia-driven PCI and 2.2% symptom-/ischaemia-driven CABG. TVF occurred in 13.9% (Table 5).
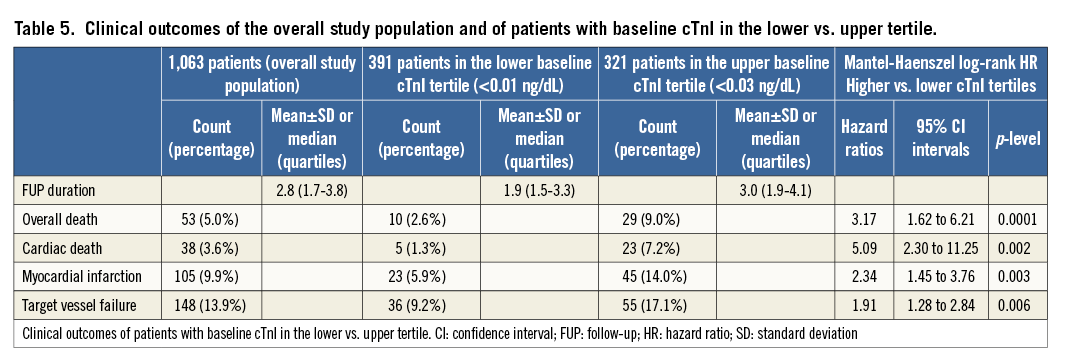
Compared with the lowest tertile, the highest tertile showed an increased risk of overall death (HR 3.17; Figure 1), cardiac death (HR 5.09; Figure 2), MI (HR 2.34; Figure 3) and TVF (HR 1.91; Figure 4).
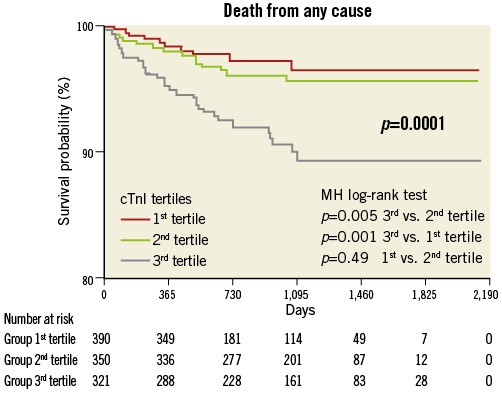
Figure 1. All-cause mortality by pre-procedural cTnI tertile.
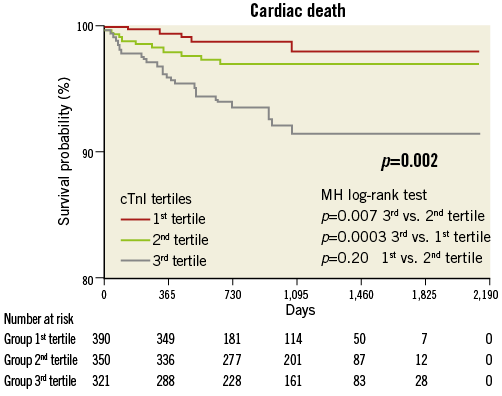
Figure 2. Cardiac mortality by pre-procedural cTnI tertile.
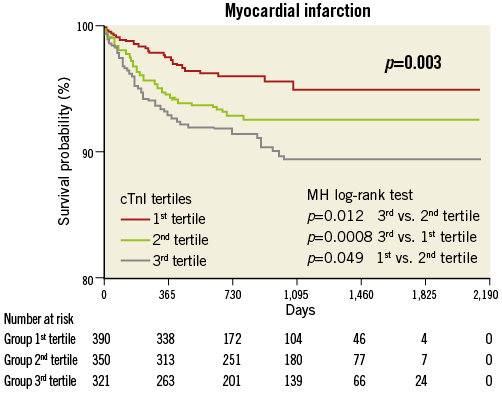
Figure 3. Myocardial infarction by pre-procedural cTnI tertile.
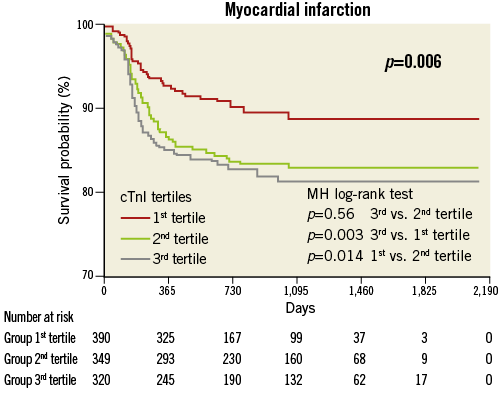
Figure 4. Target vessel failure by pre-procedural cTnI tertile.
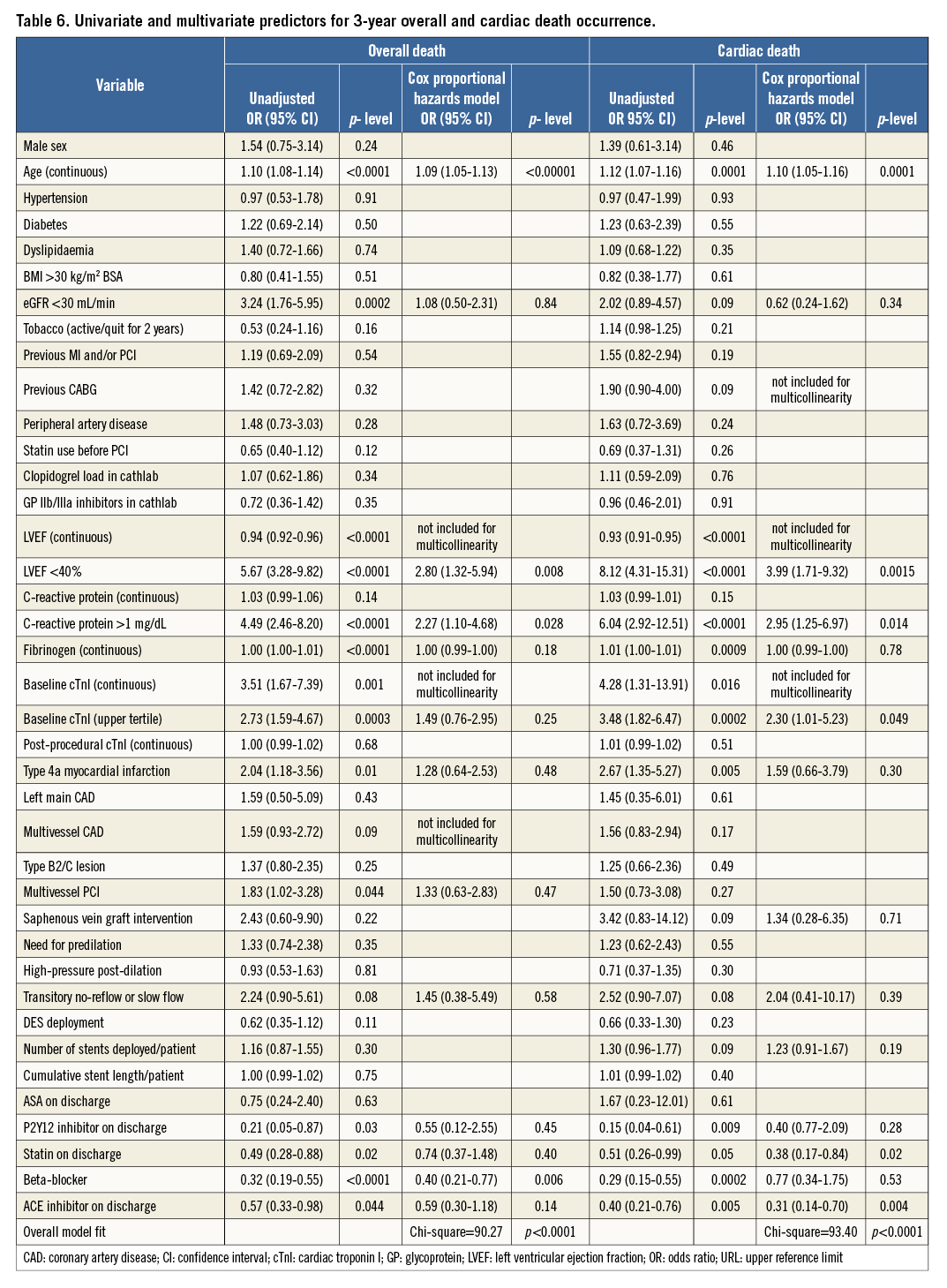
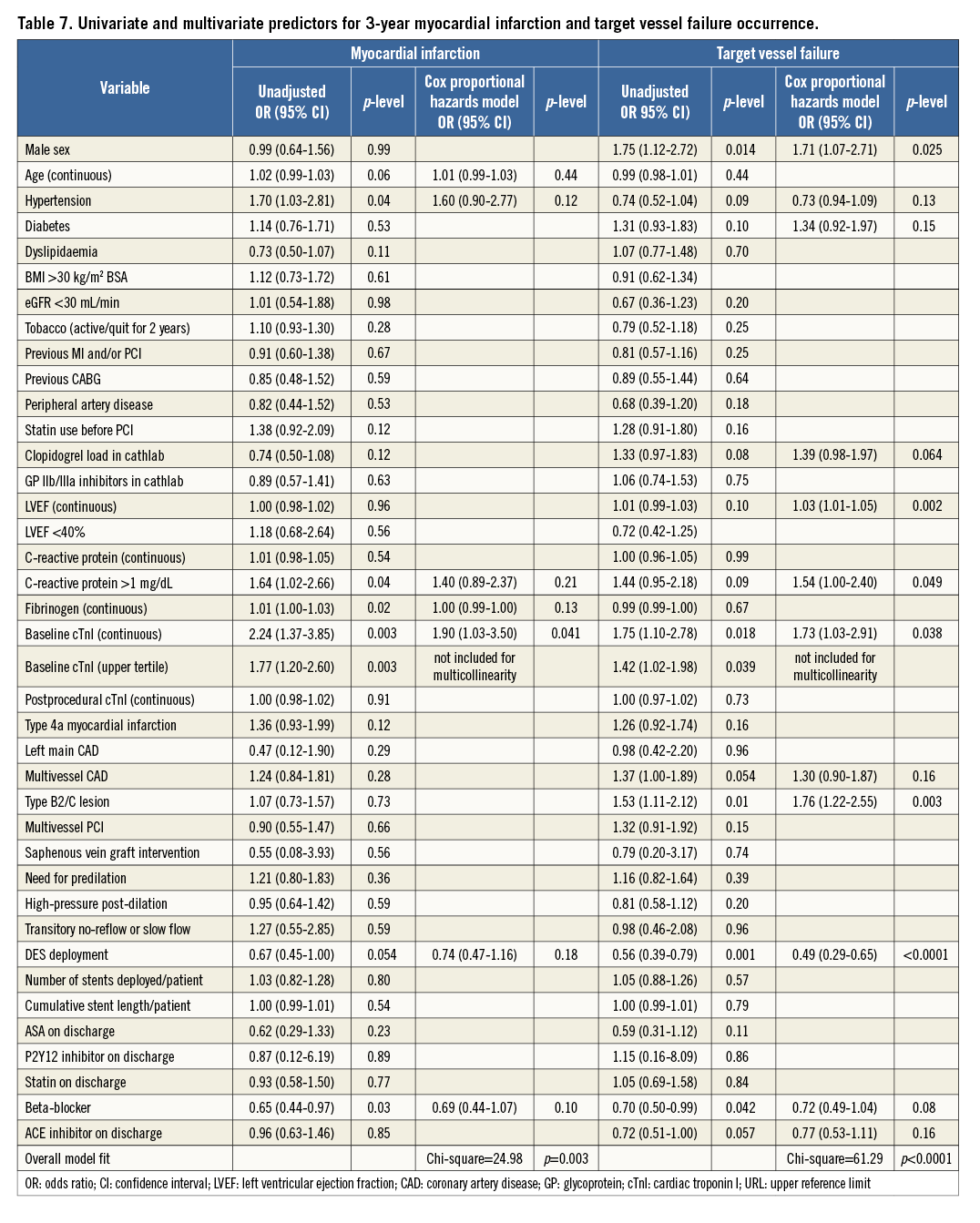
By Cox modelling, pre-PCI cTnI levels in the upper tertile were an independent predictor of cardiac death, MI and TVF, but not overall mortality (Table 6, Table 7). Other independent predictors were age, LVEF <40%, CRP levels >1 mg/dL and beta-blockers (Table 6, Table 7).
When baseline cTnI values were included in analysis of the prognostic value of the post-PCI values, the minor significance found for a fivefold increase was eliminated.
Discussion
The present study in patients undergoing PCI for stable CAD confirms the importance of pre-procedural cTnI levels even below the 99th % URL. These low values presage endpoints probably related to their association with a greater severity of coronary artery disease. These observations are similar to data in acute coronary syndromes2. The patients with cTnI in the upper tertile of the “normal range” are probably those who will have elevated cTn with high-sensitivity cTn (hscTn) assays which are associated with more extensive and more complex coronary artery disease4 and an adverse prognosis8,9,14,15. As previously, when baseline values were included in the analysis, post-procedural values did not add in identifying hard outcomes. Without scrutiny of baseline values, this observation would have been missed.
The third universal definition of MI states that to diagnose a type 4a MI the baseline value must be less than the 99th % URL and there must be increases exceeding five times the 99th percentile12. Only one published study has adhered to these criteria1. Most have used non-guideline-recommended high cut-off values for the pre-PCI value, disregarding the information embedded in the measurement16. Our data add an additional caveat. Even if normal baseline values are present, those in the upper range of normal may need to be included to provide accurate evaluation of the contribution of the baseline to prognosis.
hscTn assays have elucidated the diagnostic and prognostic importance of cTn values previously considered “normal”9,16,17. Values below the URL levels are associated with adverse events18,19, including those in stable CAD8,9,19. Until now, there has been no information with regard to PCI. In our cohort , the assay we used, as others20 relying on values below the 99th % URL, presaged risk in a similar manner to hscTn in the PEACE trial8. The assay had a 10% CV value (0.03 ng/ml) below the 99th % value. The PEACE trial data, and now ours, suggest that the 99th % URL values obtained with conventional assays are too high. Recent studies with hscTn support this hypothesis10,11. It is likely the patients in our upper tertile will have elevated hscTn values14,15,21,22.
Our study is unique in evaluating patients with stable CAD, normal pre-PCI levels and angiographic success. Thus it represents a “clean” model to study the baseline cTn values below the URL. Patients with a pre-procedural cTnI in the lowest tertile had a significantly better long-term prognosis than those in the upper tertile with lower cardiac mortality, and a reduced incidence of myocardial infarction and target vessel failure, consonant with data from the PEACE trial8, Hope15 and ARIC22. Our data unmask the complex interaction between baseline values and post-PCI values and how careful one needs to be in attributing harm to the procedure when the signal for harm is in the baseline information. Our data hint at the reasons for this. In patients with stable CAD, higher baseline hscTn values are a risk factor9. They may represent remnants of undetected episodes of coronary plaque destabilisation and/or more extensive disease4,23,24.
Finally, we observed a correlation between pre-PCI cTnI and post-procedural cTnI elevations above the URL. Without analysis of baseline values, one might have argued that a fivefold increase in cTn post PCI was prognostic. However, that effect was eliminated by analysis of baseline values. Our data are in keeping with the hypothesis that the prognostic value of cTnI is linked to the underlying CAD complexity1,4.
Study limitations
Our population was selected and thus our results cannot be extended to unselected populations. It is also true that baseline values did not presage overall mortality, only cardiac events. Finally, follow-up was done by researchers not blinded to hospital charts, in which troponin values were included.
Conclusions
Our study demonstrates that cTnI levels within the “normal range” before PCI must be accounted for to stratify prognosis following successful PCI in stable CAD patients and to avoid misinterpretation of post-PCI data.
| Impact on daily practice We now show that baseline pre-PCI values near the upper limit of the normal range are related to the complexity and severity of the underlying coronary artery disease in patients undergoing PCI for chronic stable coronary artery disease. Probably for this reason, these values manifest prognostic importance which clinicians need to be aware of. Furthermore, taking these baseline values into account eliminates the influence of post-PCI cTnI elevations. |
Conflict of interest statement
A. Jaffe consults or has in the past consulted for most of the major diagnostic companies who make cardiac troponin assays, including Siemens, the company which produces the assay used in this research. The other authors have no conflicts of interest to declare.

