Abstract
Aims: To evaluate the potential for mitral annular (MA) size reduction using a novel device utilising therapeutic ultrasound (TU).
Methods and results: The ReCor device (ReCor Medical, Inc., Ronkonkoma, NY, USA, Investigational device, not for use in human application) was studied in a closed chest canine animal model (35 dogs). Under fluoroscopy, a 12 Fr TU balloon catheter was advanced into the left atrium (transseptal approach). The TU balloon was inflated with contrast-saline, positioned at the MA and energy delivered circumferentially, to heat the tissue locally. Five TU applications were delivered (at least 60W for at least 40 sec). Relative to baseline, mitral valve annular diameter reduction (measured by transthoracic echocardiography) was 8.4% immediately post procedure(p<0.001), 8.6% at one week (p<0.001), 8.8% at two weeks (p<0.001), 9.3% at three weeks (p<0.001), 10.8% at four weeks (p<0.001), 8.6% at three months (p<0.001) and 5.7% at six months (p<0.001). Histology showed an increase in elastin associated with tissue thickening at the annular level. Transmission electron microscopy demonstrated a decrease in diameter of individual collagen fibres in treated regions compared to controls.
Conclusions: Therapeutic ultrasound (TU) energy application to the mitral annulus is feasible percutaneously. A reduction in annular dimensions occurs immediately and appears to be durable without peri-annular damage.
Abbreviations
TU: therapeutic ultrasound
ICE: intracardiac echo
LV: left ventricle
LVOT: left ventricular outflow tract
MR: mitral regurgitation
MA: mitral annulus
PMVR: percutaneous mitral valve repair
TEE: transesophageal echocardiography
Introduction
Moderate or severe mitral insufficiency is a common problem, affecting more than two million people in the United States, where it is the commonest form of valvular heart disease1; it is the second commonest indication for valvular surgery in Europe2 and results in high health care consumption and increased morbidity and mortality. The majority of mitral regurgitation (MR) is caused by a reduction or elimination of the normal systolic coaptation between anterior and posterior mitral leaflets, which normally ensures mitral competence3, and a large proportion of this is due to mitral annular (MA) dilatation4.
Preliminary animal studies have shown a reduction in annular dimension5 and in mitral regurgitation due to prolapse6 following directed radiofrequency ablation (RFA) to mitral leaflets and chordae, specifically with the QuantumCor device. The ReCor therapeutic ultrasound (TU) system (Figure 1; ReCor Medical, Inc., Ronkonkoma, NY, USA) is a steerable balloon catheter-based technology originally devised for electrophysiological ablation procedures7.
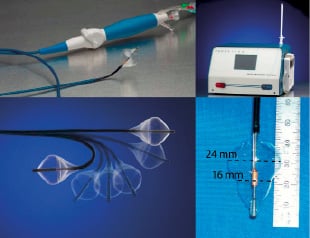
Figure 1. The ReCor device (top left) comprises a steerable catheter (bottom left) which is able to direct the actual acorn shaped balloon housing the TU emitter (bottom right). This connects to a signal generator (top right), regulating the power and duration of sonifications.
It was postulated that a modified ReCor TU percutaneous mitral valve repair (PMVR) system could be used to heat collagen in the mitral annulus sufficiently to create contraction and then reduce the overall dimension of the mitral annulus and ultimately be used to treat functional MR. We sought to test this hypothesis in vivo.
Methods
The device
The ReCor (ReCor Medical Inc., Ronkonkoma, NY, USA) percutaneous mitral valve repair (PMVR) balloon catheter was constructed with slight modifications to the catheter previously used for the ablation of atrial fibrillation. It consists of a multi-lumen shaft with a cylindrical piezoelectric ceramic transducer located towards the distal end. The PMVR catheter is deployed into and through the left atrium through a commercially available 12 Fr steerable transseptal sheath over a 0.035” guidewire, which is accommodated by the central lumen. The transducer converts electrical energy to acoustic energy, which is then delivered radially through a balloon inflated with a contrast-saline mixture at the mitral valve annulus site. The latest generation balloon (used for this study) is acorn shaped and measures 24 mm at its largest diameter and 16 mm at the level of the piezoelectric ceramic transducer (area at this level ≈2 cm2); therefore during sonifications the balloon will not be occlusive, occupying less than half of the mitral annulus area (Figure 1).
The procedure
The study was approved by the local animal ethics committee and performed in dedicated animal laboratories in Montreal and Oklahoma respectively. Dogs (30-45 kg) were studied by a closed chest approach under general anaesthesia. The procedure was performed using fluoroscopic guidance throughout and the animals were anti-coagulated with heparin. Femoral venous access was used to facilitate conventional transseptal puncture to access the left atrium. The transseptal catheter was exchanged over a 0.035” long exchange wire for a customised 12 Fr deflectable transseptal sheath. The 0.035” exchange wire was advanced to the LV with the help of a balloon tip catheter (Critikon, Tampa, FL, USA) and positioned across the aortic valve and aortic arch into the descending aorta.
The PMVR catheter was advanced over the wire through the transseptal sheath using the deflection function to adjust orientation; the tip of the catheter was advanced into the mitral valve so that the transducer was at the annulus and the balloon inflated. Position of the transducer relative to the mitral annulus was ensured in two orthogonal views by X-ray fluoroscopy (Figure 2) or intracardiac/transesophageal echocardiography (ICE/TEE).
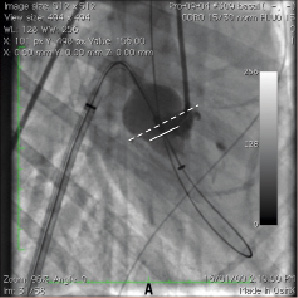
Figure 2. Procedural fluoroscopy showing a sheath across the interatrial septum: the steerable catheter is deflected over the wire towards the mitral annulus. A. Right anterior oblique projection. B. Left anterior oblique projection. Contrast is seen in the posterior groove delineating the mitral annulus (dashed line delimits the MA, plain line the waist of the acorn balloon at the level of the piezoelectric ceramic transducer).
Electrocardiogram and blood pressure were monitored closely during balloon inflation and especially during sonifications. Each sonification delivered TU energy (9 MHz) circumferentially (perpendicular to the catheter shaft) to heat the local tissues. A total of five applications (at least 60 watts for at least 40 seconds) per animal were planned. Coronary angiography was performed before and after the procedure to ensure no coronary compromise, particularly of the circumflex.
Echocardiography
Transthoracic 2-D echocardiogram and Doppler continuous wave imaging were performed to measure the MA diameter in end diastole (in apical four-chamber, three-chamber and two-chamber views) and mitral flow before, immediately after, and 1, 2, 3 and 4 weeks, 3 months and 6 months postprocedure. This provided mitral annular dimensions in inferoseptal-lateral (apical 4-chamber view), antero-inferior (apical 2-chamber view) and anteroseptal-posterior (apical 3-chamber view) projections; the maximal and minimal dimensions of the mitral annulus measured in these projections were recorded at each time point. Presence and degree of mitral regurgitation were assessed using a semi-quantitative method at each time point8. Echocardiograms were performed by an experienced physician, not present during the procedures and blinded to the power and duration of sonifications. Sequential echocardiograms were performed in all animals acutely (periprocedurally) and chronically in a subset of 20 animals over four weeks; nine animals were followed for the full six months. Eight animals have been sacrificed sequentially for tissue analysis.
Gross and histopathological analysis
Gross and microscopic analysis was performed at the CV Path Institute, Inc. (Gaithersburg, MD, USA). Explanted hearts from dogs sacrificed at various time points were all bisected along the short axis to isolate the base of the heart. Then the base of the heart for each animal was transected longitudinally across the P3 cusp of the posterior leaflet to expose the left atrium and mitral valve apparatus. The mitral valve leaflets and subvalvular apparatus, annular tissue and adjacent atria and ventricle, as well as the coronary arteries were specifically studied in detail for injury.
The mitral valve was sectioned to include representative slices from the three posterior scallops (P1, P2, P3) of the posterior leaflet and corresponding anterior leaflet regions (A1, A2, A3). Each representative slice encompassed a portion of atrium, the mitral annulus, the attached leaflet and adjacent ventricular myocardium. These tissue slices were dehydrated in a graded series of ethanol and embedded in paraffin. The paraffin blocks were cut on a rotary microtome at 4-5 microns, mounted on charged slides and stained with haematoxylin-eosin (H&E), Movat pentachrome and Masson’s trichrome stains. Transmission electron microscopy (TEM) was performed on deparaffinised tissue stained with uryl acetate/lead citrate using a Hitachi 8600 transmission electron microscope and images were photographed using an AMTV542 camera system.
Statistical methods
Statistical analyses were performed using SPSS software (SPSS Inc, Chicago, IL, USA). Differences were assessed using a paired sample T-test (normally distributed data). For correlations, a Pearson bivariate analysis with a p<0.05 by two tailed was considered significant (parametric variables).
Results
Procedural results
Thirty-five dogs were studied in total (33 procedure subjects and two control sham procedures). Of the 33 treated subjects, 29 underwent five sonifications each; to test safety of multiple sonifications one dog underwent eight sonifications. The first subject studied represented the initial technical feasibility case with this device and underwent only three successful sonifications; he was sacrificed early for pathologic analysis without any measurements being performed. Each sonification was delivered for a mean of 45.3±19.3 (40-120) seconds with delivered power at 84.4±28.8 (60-130) watts, to gauge a dose response. Pulmonary pressures were monitored throughout the procedure in the first 10 dogs and aortic pressures in all dogs. There was no change in these pressures observed during balloon inflations.
One animal died from anaesthesia-related complications. Another death was due to ventricular fibrillation refractory to cardio version, following the second high frequency energy delivery; this was the only device-related death. There was no heart block seen. Another dog suffered a retroperitoneal haematoma related to femoral arterial access necessitating early sacrifice.
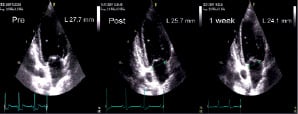
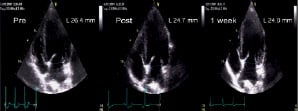
Figure 3. Representative changes in mitral annular dimension demonstrated by echocardiography over time. A. Antero-inferior dimension shown in the apical 2-chamber view. B. Septolateral dimension shown in the apical 4-chamber view.
Impact on mitral annular size
There was an acute reduction observed in both maximal and minimal measured mitral annular dimension on echocardiography. This reduction was instantaneous and sustained with little additional change at later time points (Figure 4, Table 1).
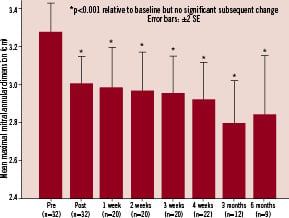
Figure 4. Mitral annular dimension over time. An immediate reduction in mitral annular dimension is observed which is sustained over six months.
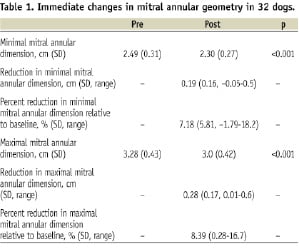
Relative to baseline, mitral valve annular diameter reduction (based on maximal dimension) was 8.4% immediately postprocedure (n=32, p<0.001), 8.6% at one week (n=20, p<0.001), 8.8% at two weeks (n=20, p<0.001), 9.3% at three weeks (n=20, p<0.001), 10.8% at four weeks (n=22, p<0.001), 8.6% at three months (n=12, p<0.001) and 5.7% at six months (n=9, p<0.001). Based on the minimum mitral annular diameter measured, mean annular reduction relative to baseline was 7.2% immediately post procedure (n=32, p<0.001), 8.6% at one week (n=20, p<0.001), 9.6% at two weeks (n=20, p<0.001), 9.9% at three weeks (n=20, p<0.001), 8.6% at four weeks (n=22, p<0.001), 6.3% at three months (n=12, p<0.001) and 9.6% at six months (n=9, p<0.001). The reduction in mitral annular diameter dimension appeared sustained at six months (Figure 4). There was a clear dose response in mitral annular reduction according to power delivered per sonification and also duration of each sonification (Figure 5).
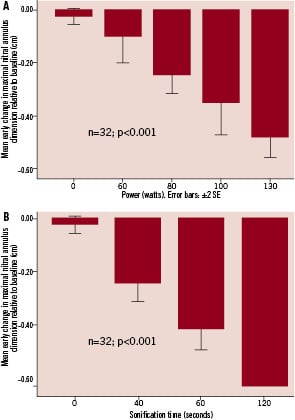
Figure 5. Dose response in mitral annular reduction according to power and duration of sonifications.
In a multivariate linear regression model for absolute immediate reduction in mitral annular dimension with power and duration of sonification as the dependent variables only power was an independent predictor of mitral annular reduction (p<0.001 for maximal dimension, R=0.665; p=0.001 for minimal dimension, R=0.556).
Mitral valve function
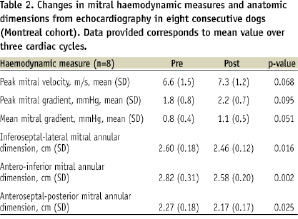
Mitral valve E and A velocities, peak and mean gradients were measured in eight consecutive dogs (Montreal cohort) using continuous wave Doppler (CWD). In parallel with a reduction in mitral annular dimensions, there was a slight increase in these gradients, without causing any haemodynamically significant stenosis (Table 2). Two animals had moderate mitral (2+) regurgitation at baseline, with an acute reduction to mild MR (1+) after TU treatment.
Gross and histopathological data
Acute (7-day) animals
There were two 7-day (acute) animals, which on gross examination showed focal erythema on the atrial walls with extension onto the anterior leaflet in one animal and both anterior and posterior leaflets (cusps A1, A2, P1 and P2) in the second animal. On microscopic examination, both hearts exhibited focal fibrin deposition and thickening on the anterior and posterior atrial surfaces.
At the level of the mitral annulus, sections from both animals demonstrated coagulated collagen and contraction band (myocyte) necrosis, focally extending into the ventricular myocardium in one animal. These areas showed extravasated red cells, acute and chronic inflammatory cell infiltrates, oedema and fat necrosis. In both animals, sections from the leaflets demonstrated extravasated red blood cells in the atrialis layer with increased proteoglycan deposition in the spongiosa layer, predominately affecting the A2 and P1 cusps.
Chronic (six month) animals
In the six chronic animals, multiple areas of endocardial fibrosis and/or thickening were grossly identified on the atrial and mitral annular surfaces without significant thickening of the mitral leaflets. Corresponding microscopic sections from the animals exhibited mild to moderate endocardial fibroelastosis of the atrial wall and at the level of the mitral annulus. Focal calcification associated with fibroelastosis was identified in the atrial wall in one animal. Four of the six animals exhibited changes of the mitral valve leaflets including focal mild fibroelastosis of the atrialis layer (Figure 7), increased proteoglycan deposition in the spongiosa layer and chondroid metaplasia with focal calcification. In all six of the chronic animals, the coronary sinus and left circumflex coronary artery were unremarkable, as well as the ventricular myocardium with only a few foci of endocardial thickening.
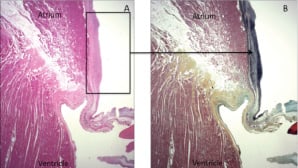
Figure 7. Histopathology of the mitral valve posterior leaflet. Endocardial thickening is seen on the atrial surface directly superior to the mitral annulus. The mitral annulus and posterior leaflet of the mitral valve are unaffected. A: H&E stain, B: Movat stain, showing increased elastin fibres (black staining) at the area of endocardial thickening (arrow).
Transmission electron microscopy
Transmission electron microscopy was performed at the level of the mitral annulus in two acute and two chronic animals, all with similar changes in collagen diameter in the treated areas. Both seven day animals demonstrated a decrease in the normally observed axial periodicity of collagen fibres, increased compaction, and a decrease in diameter of individual fibres (average diameter of treated collagen 0.044±0.008 µm and 0.058±0.069 µm versus 0.065±0.007 µm and 0.064±0.004 µm in control sections, in animals one and two, respectively), which contribute to annular reduction (Figure 8).
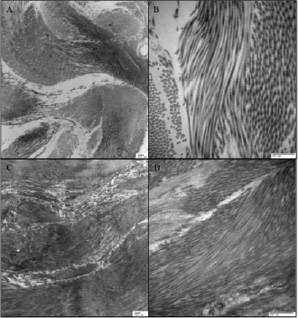
Figure 8. Transmission electron microscopy images taken at the level of the mitral annulus adjacent to the P1 segment of the mitral apparatus in control areas (A and B) and affected area (C and D) at low (10,000X) and high magnification (40,000X), respectively. Both low and high power images demonstrate a decrease in collagen fibril diameter in the treated area (average diameter 0.044±0.008 µm) with respect to collagen in the unaffected tissue (average diameter 0.065±0.007 µm), as well as loss of the normal axial periodicity in the treated collagen fibres.
Similar findings were seen at the level of the mitral annulus in two chronic animals (average diameter of treated collagen 0.0430±0.007 µm and 0.0478±0.005 µm versus 0.070±0.007 µm and 0.0670±0.008 µm in control animals, in two separate animals, respectively).
In summary, the acute animals demonstrated histologic evidence of collagen coagulation at the level of the mitral annulus, with a decrease in collagen fibre diameter. The chronic animals exhibited fibroelastosis in the atrial wall and annular region, microscopic foci of chondroid metaplasia and/or calcification in the atrial wall and mitral leaflet. All these changes were considered within the normal limits of physiologic reactions to instrumentation in this species.
There was a strong correlation observed between the location of energy deposition (as estimated under fluoroscopy) and the location of the pathological findings. There was one chronic heart with injury at the posterior papillary muscle, which also showed thickening at the anterior leaflet, but this was attributed to contrast injections into the mitral valvular apparatus periprocedurally with a JR4 catheter inserted retrograde across the aortic valve (Figure 2), which was employed in a few subjects in lieu of ICE/TEE. There was no other damage observed.
Discussion
We have demonstrated in this preclinical study that therapeutic ultrasound is safe and effective in reducing annular dimension, and affords results that are sustained at six months.
Feasibility, ease of use and reproducibility
We have shown the technique of TU to the mitral valve to be feasible. Its notable ease of method owes a lot to its similarity to the well established procedure of percutaneous transluminal mitral valvuloplasty. Moreover, there is the potential for repeat procedures with possible additive effects with this strategy. There are similarities to the QuantumCor device5 in terms of energy delivered to the mitral annulus to facilitate shrinkage. However, the ReCor device differs in the use of high frequency ultrasound, which is focused and does not require contact, as opposed to radiofrequency energy in QuantumCor which requires contact; this makes the latter arguably a more challenging device to use with the need to steer the catheter to several positions. An advantage in relation to the coronary sinus approach is that this technique targets the mitral annulus directly, whereas the coronary sinus is heterogeneously associated with the mitral annulus, coursing superiorly to the latter in the majority of cases9. Moreover, in 70% of patients the coronary sinus overlaps a coronary artery with risk of compression at the time of annuloplasty10.
Efficacy, pathology, safety
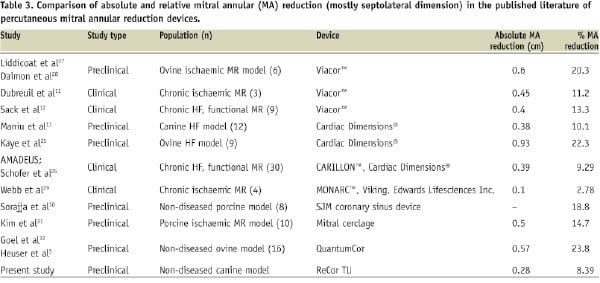
TU achieved an immediate mitral annular diameter reduction of 2-3 mm, on average. This is comparable to the experience of devices such as Viacor™ in clinical studies and Cardiac Dimensions® in canine preclinical and clinical studies11-14; both of these coronary sinus based annuloplasty devices have been recently shown to successfully attenuate functional mitral regurgitation in early clinical studies12,14 (Table 3).
Reductions of up to 6 mm were seen in subjects receiving the highest power sonifications.
Heating collagen-rich tissue to sub ablative temperatures causes shrinkage. This phenomenon has been used for some time in the treatment of widespread diseases of collagenous tissues, including thermokeratoplasty in the eye15 and therapies for joint instability16. The mitral valve annulus (MA) is composed mainly of collagen, and its synthesis has been shown to be unregulated in experimental models of mitral regurgitation17, hence it seems an excellent target to decrease mitral annular dimension and MR.
TU was first used in clinical settings in the 1950s in the field of neurosurgery18, but only more recently –with the advance of imaging technologies to guide its delivery– has its use become widespread with growing applications in the fields of urology, oncology and gynaecology to name but a few, all capitalising on its ability to exert a relatively well circumscribed area of scarring19-21. We observed this phenomenon using TU to the mitral valve annulus, causing a well circumscribed area of injury localised to the annulus and documented in histopathological specimens, with adequate sparing of the mitral valve leaflets and apparatus.
Comparison of results seen with current mitral annular reductive therapies (Table 3)
Numerous device strategies have emerged to treat mitral regurgitation by a transcatheter approach22. To date, those that have dominated include edge-to-edge repair23,24 and coronary sinus approaches. These involve implantable devices and have demanded new skills from the interventionalist, as well as prolonged procedure times. In contrast, TU for mitral annular reduction offers a simpler approach, albeit requiring transseptal catheterisations skills, with no need for a device to remain in situ.
The 8.39% mitral annular diameter reduction seen here with TU may at first glance appear smaller than that seen in other preclinical research studies with novel percutaneous devices designed to achieve this endpoint and may not translate in clinical improvement (Table 3). However, the degree of mitral annular reduction seen with devices in preclinical studies appears related to the animal model used, with ovine and porcine models often achieving a greater annular reduction for the same device than with canine models and clinical studies (Table 3). For instance, the Cardiac Dimensions® device achieved a 22.3% mitral annular reduction in an ovine model, 10.1% in a canine model and 9.29% in the recently published AMADEUS clinical trial13,14,25. It is conceivable that TU may achieve even more favourable results when this technology enters clinical studies with dose titration, as higher power and longer duration sonifications achieved a mitral annular diameter reduction of more than 18% in the canine model. We saw evidence of tissue contraction at P1 A1 and to some extent at P2 A2 (Figure 6), which was a function of positioning of the therapeutic ultrasound balloon. It is conceivable also that delivering energy to more locations along the annular circumference, through angulation of the balloon shaft during/between sonifications, may achieve an even greater annular reduction.
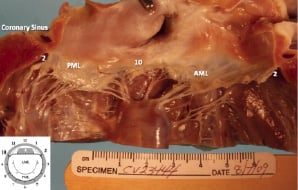
Figure 6. Macroscopic pathology showing scarring at the level of the mitral annulus with preservation of the mitral valve apparatus. Numbers correspond to surgical clock (bottom left). AML: anterior mitral leaflet. PML: posterior mitral leaflet. There is focal endocardial fibrosis beneath the PML.
Dose responsiveness
There was a clear dose responsiveness with both power and duration of sonifications for TU to exert mitral annular reduction (Figure 5). As well as providing evidence for the effectiveness of this novel therapy, this suggests that tailored treatment using progressive titrations of power and duration of sonifications would be feasible in a clinical setting, with the possibility of repeated procedures –if necessary– in a manner analogous to percutaneous transcatheter mitral valvuloplasty. Importantly, even at the highest power (130 watts) and longest duration (120 seconds), there was no evidence of mitral valvular leaflet injury. Remarkably, the histopathological data available (Figure 7) demonstrates that the scarring induced was restricted to the annulus itself, with sparing of the mitral valve apparatus. This is probably related to the fact that the annular tissue remains in a relatively fixed position during the cardiac cycle or is in phase with the catheter, enabling the ultrasound waves to converge and deliver a circumferential ring of controlled injury. In contrast, the mitral valve apparatus is a more dynamic target and this may avoid contact with converging ultrasound waves that is sufficiently prolonged to cause injury.
Study limitations
This experiment evaluated the ability of a catheter based system to reduce mitral annular dimension, with the ultimate goal of treating functional mitral regurgitation in humans in the setting of heart failure. One important limitation was that the vast majority of the animals studied did not have mitral regurgitation and thus the ability of this device to treat this disease entity could not be comprehensively evaluated. However, this was also the case for the preclinical evaluation of the Evalve MitraClip™ edge-to-edge based mitral valve repair26, which has since become the first percutaneous mitral valve repair device to attain CE (Conformité Européenne) mark for mitral regurgitation, with the subsequent promising results of the EVEREST study23.
Conclusion
Therapeutic ultrasound (TU) energy application to the mitral annulus is feasible percutaneously. Importantly, our study demonstrates the capacity for TU to safely reduce annular dimension and compares favourably to transcatheter devices already applied to reduce mitral regurgitation13,14. In a canine model, annular contraction occurs immediately and appears to be sustained without peri-annular damage.

