Introduction
The Carillon Mitral Contour System® (Cardiac Dimensions, Kirkland, WA, USA) is a CE-marked percutaneous device for the treatment of functional mitral valve regurgitation (FMR) in systolic heart failure.
Device description
Briefly, two self-expanding nitinol anchors connected by a nitinol curvilinear segment are introduced into the coronary sinus (CS). After unsheathing the distal anchor, tension is applied to the system resulting in an anterior movement of the posterior mitral leaflet. The proximal anchor is then deployed near the CS ostium to fix the tension (Figure 1).
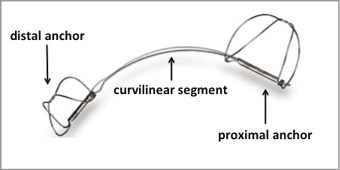
Figure 1. Carillon Mitral Contour System®.
Although the procedure could be done under deep conscious sedation, general anaesthesia is favourable due to continuous transoesophageal echocardiography guidance during device implantation. After right femoral artery puncture, coronary artery angiography is performed for visualisation of the circumflex coronary artery. The guiding catheter remains in the LCA ostium during the implantation. This allows for the possibility of a rapid LCA angiography at any time during the procedure.
Venous access is obtained with a 10 Fr sheath in the right internal jugular vein. A 7 Fr multipurpose catheter is introduced into a Carillon 9 Fr delivery catheter and placed before the CS ostium. A hydrophilic-coated 0.035’’ guidewire is then delivered via a multipurpose catheter into the CS and positioned in the great cardiac vein. The delivery catheter is moved over the multipurpose catheter and the wire moved up into the anterior interventricular branch of the great cardiac vein.
SIZING
After removal of the guidewire and the multipurpose catheter, a venography for confirmation of the correct CS position is performed with a marker catheter (Carillon 5 Fr) placed in the delivery catheter. This allows sizing of the coronary sinus and of the Carillon device. Thirty-seven different combinations of distal (7-14 mm) and proximal (16-20 mm) anchor diameters and device lengths (60, 70 and 80 mm) are available. Venous characteristics (side branches, tapering) and the relationship of the CS to the circumflex coronary artery should be taken into account while choosing the appropriate device. In order to pull at least 3 cm of tension, the length of the vein should account for at least 9 cm (using a 60 mm long device) between the distal landing zone and the CS ostium.
PLACEMENT OF THE CARILLON DEVICE
After withdrawal of the marker catheter, the appropriately sized Carillon device is introduced into the delivery catheter. Under fluoroscopic guidance the distal anchor is unsheathed in the target location within the great cardiac vein and locked. Afterwards, tension is applied to the system to move the posterior part of the mitral annulus towards the anterior and thereby allow the mitral leaflets a better coaptation. This acute effect on FMR is documented by echo. Regurgitant volume, effective regurgitant orifice area (EROA), vena contracta and annular septal-lateral diameter should be reduced due to device implantation.
The proximal anchor is then positioned near the CS ostium and fixed. Repeat left coronary angiography should be performed to rule out circumflex artery obstruction. Re-sheathing is mandatory when coronary artery obstruction occurs with signs of ischaemia in the ECG even after intracoronary nitroglycerine injection. After FMR assessment and when the left and right coronary artery angiography shows no coronary obstruction, the Carillon device can be released (Figure 2).
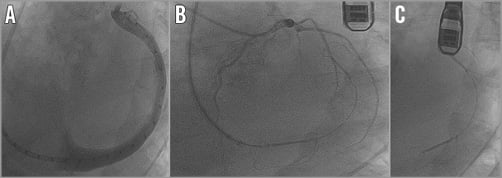
Figure 2. Carillon implantation steps. A) Coronary sinus venography (LAO projection) with the Carillon 5 Fr marker catheter in the 9 Fr delivery catheter. B) Coronary angiography of the LCA after Carillon Mitral Contour System® implantation (LAO projection). C) Released Carillon Mitral Contour System® in the coronary sinus.
Patient selection
Optimal patient selection prior to Carillon implantation is mandatory to achieve positive clinical results with this technology. Table 1 summarises the main aspects for a safe and effective Carillon implantation. Patients with symptomatic (>NYHA II) dilated ischaemic or non-ischaemic cardiomyopathy (left ventricular ejection fraction <40%) on stable heart failure medication (according to the guidelines) suffering from at least moderate, ideally pure, FMR are eligible for percutaneous annuloplasty. In patients with massive left atrium dilatation and severe FMR the likelihood of marked efficacy might be reduced. Understanding that FMR is dynamic (worsened by exercise), patients with FMR grade 2 and 3 are likely to experience FMR reduction with associated symptom improvement.
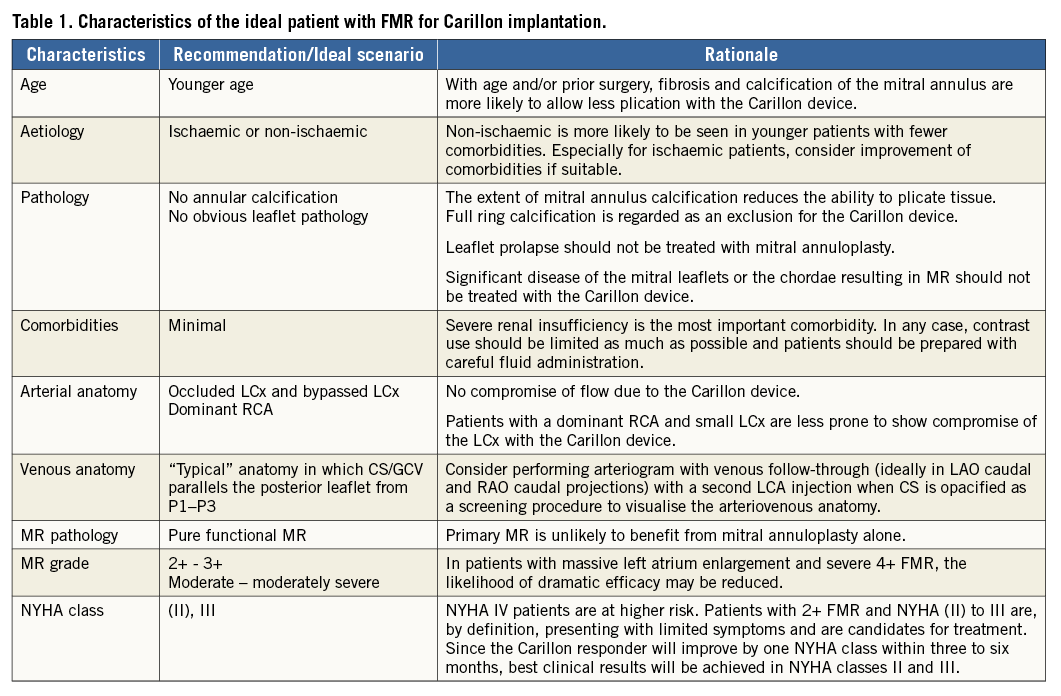
The goal is to optimise the ability to plicate peri-annular tissue. With age or prior surgery fibrosis and calcification are more likely. Therefore, patients aged <65 years are ideal for mitral annuloplasty. Patients with significant organic mitral valve pathologies or severe mitral annular calcification should be excluded. Leaflet prolapse should specifically not be addressed with the Carillon device. Therefore, echocardiographic assessment (transthoracic and transoesophageal) of the mitral valve according to the guidelines is mandatory prior to scheduling Carillon device implantation.
Evaluating the results
Determination of regurgitant volume, proximal isovelocity surface area, effective regurgitant orifice area (EROA), vena contracta and annular septal-lateral diameter are recommended to monitor effectiveness after the implant. Three-dimensional (3D) echocardiographic evaluation is helpful to evaluate the anatomy and pathology of the mitral valve. Rendering 3D data also helps understanding the anatomical relation between the coronary sinus and the mitral annulus.
Of note, between responders and non-responders (measured as reduction in FMR), no difference in the CS or great cardiac vein position of the device relative to the mitral annulus could be obtained in cardiac computed tomography (CT) scans. However, 3D echocardiography data include the mitral annulus motion and could also be performed after Carillon implantation to learn more about positioning of the device and its efficacy. This feature is currently under investigation.
Contrast-enhanced CT scanning as a screening procedure for patient selection and reliable coronary sinus sizing is currently limited because of insufficient complete opacification of the coronary sinus after intravenous contrast injection.
In the case that coronary angiography is performed before a planned percutaneous mitral valve intervention, the CS should be visualised by a contrast run through injection into the LCA with a prolonged cine recording and a second coronary angiography when the CS is opacified. While obstructing the circumflex coronary artery by the device could complicate the Carillon implantation, this manoeuvre is useful in order to understand the relationship between the circumflex coronary artery and the CS. However, the device could be recaptured in case of coronary artery obstruction. This allows deploying a new device in another position, preventing obstruction of the circumflex coronary artery. According to our current experience, in 10 to 15% of cases, the implantation of a Carillon device is not possible because of anatomical interaction resulting in LCx obstruction.
Although contrast medium at reasonably low volume is required for Carillon implantation, severe renal insufficiency should be noticed prior to implantation in order to avoid acute post-procedure renal failure. Therefore, contrast agent usage should be minimised as much as possible. It should be noted, however, that successful percutaneous mitral valve interventions can improve renal function.
Discussion
Of note, the Carillon device can be implanted prior to a cardiac resynchronisation therapy (CRT) device in heart failure patients with wide QRS complex and FMR. Explant of left ventricular CRT lead and reintroduction after Carillon implantation is possible but technically challenging (if not currently implanted) and should only be a bailout strategy in highly symptomatic patients, where other percutaneous devices for FMR are not applicable.
During follow-up of Carillon patients, a further improvement of FMR was seen both in the TITAN trial as well as in general clinical use trials. This phenomenon could be explained by a delayed decrease in the mitral annulus due to the constantly applied traction deployed by the device together with remodelling of the ventricle by reduction of end-diastolic and end-systolic dimensions. Therefore, in patients without marked acute effects on FMR during the procedure (acute non-responders), it could also be beneficial to complete the Carillon device implantation and wait for remodelling. Further studies are needed to verify this strategy.
After successful Carillon device implantation, a close follow-up is useful to identify non-responders. In case of persisting symptomatic FMR after an observational window of three to six months, transcatheter edge-to-edge mitral valve repair could be an option for treatment. Theoretically, reduction of the annular septal-lateral diameter could be beneficial for edge-to-edge mitral valve repair and deploy synergistic effects. Especially when poor coaptation prevents the grabbing of both mitral leaflets with the MitraClip, mitral annuloplasty could help to achieve better adaptation. Further clinical trials are warranted to test this combined strategy.
Conclusion
In summary, the Carillon Mitral Contour System is a reliable option in case of increased mitral annular diameters associated with pure FMR. High-quality echocardiography is necessary for primary evaluation, periprocedural management and follow-up of these patients. This allows for optimal patient selection and improves clinical results. Future perspectives, such as pre-interventional non-responder prediction or synergistic percutaneous mitral valve interventions, could broaden the use of Carillon mitral valve therapy. The Carillon device implantation procedure has a favourable acute and long-term safety profile and is associated with low major adverse events. Furthermore, it demonstrates a significant reduction in FMR in heart failure patients, and results in left ventricular reverse remodelling and improved clinical symptoms.
Several clinical trials have demonstrated the safety and positive impact on the clinical course for mitral annuloplasty with the Carillon device by reducing the degree of mitral regurgitation (AMADEUS, TITAN, TITAN II). The REDUCE FMR trial is an ongoing, randomised, double-blind study to further evaluate the safety and efficacy of the system in a planned cohort of 120 patients with symptomatic FMR. It will help to direct the future perspective of the device.
Conflict of interest statement
M. Haude and H. Degen are proctors and consultants for Cardiac Dimensions. D. Rottländer has no conflicts of interest to declare.

