Device description
Name and manufacturer: CARILLON® Mitral Contour System® (Model XE2); Cardiac Dimensions, Inc., Kirkland, WA, USA.
Approval status: CE mark approval (Model XE2) 2011. Not approved outside Europe and the Middle East.
Platform: Nitinol and titanium.
Specific design: Two self-expanding nitinol anchors with nitinol curvilinear connecting segment and titanium crimp tubes (Figure 1).
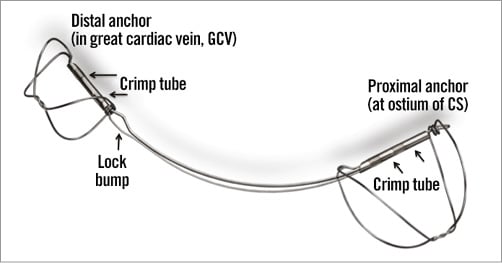
Figure 1. CARILLON® Mitral Contour System®
Delivery system: 9 Fr proprietary curved delivery catheter. The device is pre-packaged in a cartridge, which can be attached to the delivery catheter, and is connected to a delivery handle with three control knobs for delivery and release of the device, including a safety catch to be released prior to decoupling of the device.
Device sizes and lengths: A combination of distal anchor diameter, proximal anchor diameter and device length. There are two basic lengths, 60 mm and 80 mm. However, for the two largest distal anchor sizes, there is a 70 mm length. Distal anchor sizes range from 7 to 14 mm. Proximal anchor diameters range from 12 to 20 mm.
Procedural details
Venous access is obtained with a 9 Fr sheath in the right internal jugular vein. The coronary sinus is engaged and the 9 Fr curved delivery catheter is inserted up to the anterior interventricular vein branch of the great cardiac vein. A marker catheter is inserted into the delivery catheter and venography is performed (Figure 2A). Coronary arteriography is also performed to assess the relationship between the circumflex coronary artery and the coronary sinus/great cardiac vein. Using the marker catheter as a scaling device, measurements are made on the fluoroscopy screen at the intended site of the distal and proximal anchors. Anchor locations are decided upon based upon venous characteristics (e.g., favourable versus unfavourable tapering, location of side branches), coronary artery relationship, and vein length. In order to pull a minimum of 3 (preferably 4-5) cm of tension, a 9 (preferably 11) cm length of vein is required (using a 60 mm long device). For longer veins, an 80 mm long device may be selected. The fluoro screen is marked for the desired placement of the anchors. The marker catheter is removed and the appropriately sized device is inserted and advanced without fluoro to near the end of the delivery catheter, using the first control knob. Under fluoro (Moving image 1), the distal anchor is unsheathed, while pulling gently back on the delivery catheter, in order to position the distal anchor precisely, based upon the mark on the fluoro screen (Figure 2B). Upon release, the distal anchor is then locked, by reversing the direction of the control knob, using the delivery catheter to push a locking loop over a lock bump. Additional unsheathing of the device is done using the first control knob, and then tension is applied to the system by pulling the delivery catheter until the proximal aspect of the proximal anchor crimp tube is pulled to the mark on the screen designating the desired location of the proximal anchor (often at the coronary sinus ostium). Left coronary angiography is done to check for circumflex artery compression. If none, the proximal anchor is released by first continuing to unsheath as before with the first control knob, then pushing out and locking the anchor using the second control knob, which is attached to a pusher catheter inside the system (Figure 2C). Left and right coronary angiography are done to ensure patency of the vessels and, if satisfactory, the device may be released using the third control knob, after releasing the safety catch. If there is coronary compromise, the device can be recaptured by reversing direction of the first control knob and providing corresponding gentle compression on the delivery catheter in order to resheath the device (Moving image 2).
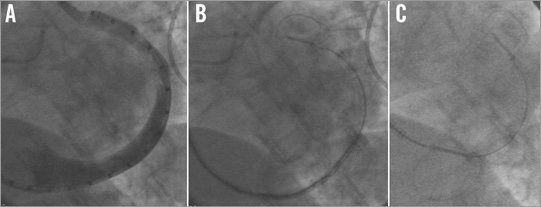
Figure 2. Fluoroscopy images. A) Coronary sinus/great cardiac vein venogram with marker catheter. B) CARILLON device with distal anchor deployed and locked in the distal aspect of the great cardiac vein. C) CARILLON device now with proximal anchor deployed at the osmium of the coronary sinus. Compare the arc of the curvature of the vein in A and the CARILLON device in B, to the new arc in C. This change in arc represents the cinching force of the CARILLON device on the posterior mitral annulus.
Clinical data
There have been three safety and efficacy studies in Europe, using different (minor) modifications of the device. In the AMADEUS1 trial, which enrolled 48 patients with symptomatic congestive heart failure, depressed left ventricular function, dilated left ventricles and moderate to severe secondary mitral regurgitation (MR), thirty patients received CARILLON devices, with the other 18 having access-related dissections, temporary coronary compromise, insufficient reduction in MR or device slippage (all of which were reduced in future studies). There were three small non-Q-wave myocardial infarctions and one death three weeks after implant related to worsening renal failure (rapidly deteriorating prior to the procedure) and heart failure. None of these complications was deemed to be device-related by an independent safety and monitoring board. Because of early device slippage, the distal anchor was modified, adding a twist, to provide more robust anchoring. Improvements over six months were seen in quantitative echo assessments of MR, as well as in six-minute walk tests (6 MWT), New York Heart Association (NYHA) classification and measures of quality of life.
The device was modified again for the TITAN2 trial, by adding a twist to the proximal anchor. This study, with a similar trial design, enrolled 53 patients with 36 receiving permanent implants. The other 17, who had devices placed and then removed, were followed for one year, as were the implanted patients. There was only one major adverse event (MACE) at 30 days in this group, again a patient death related to renal failure, providing a MACE rate of <3%. Compared to baseline, and the non-implant group, there were significant improvements in MR (Online Figure 12), NYHA class, and 6 MWT, as well as favourable LV remodelling, with the MR improving over time. Asymptomatic, and clinically silent, wireform fractures were seen in ~25% of devices, primarily at a specific location at the proximal anchor locking mechanism. The fractures were clinically silent and not associated with any adverse events, and patients with fractures had similar benefits to patients without fractures. Since it was observed that MR improved over time (Online Figure 1), the decision within the trials to remove devices based upon inadequate MR improvement at the time of implant was found to be unnecessary.
After identifying the area of high strain in the device at the specific location implicated in the fractures, the device was further modified in order to reduce the strain forces. This device was used in the TITAN II trial (submitted for publication), again with a similar patient population and trial design to the two prior studies. In this study, there was only one fracture (in a device used outside of the indications for use). Similar safety profile and improvements in MR, left ventricular remodelling, NYHA class and 6 MWT were seen to those in the TITAN trial. This device has been used in over 300 commercial implants with results seemingly comparable to those seen in the trials.
Ongoing studies
The REDUCE FMR trial is a randomised, double-blind study comparing CARILLON device implantation to guideline-directed medical therapy in patients similar to those in the prior studies. The primary endpoint is one-year improvement in mitral regurgitant volume in the treated group compared to the medically managed group. It is being conducted in several European countries and in Australia, and 120 patients are planned for the study, using a 3:1 randomisation scheme.
The Clinch study is a small pilot randomised trial in Germany, randomising patients to CARILLON versus MitraClip® (Abbott Vascular, Santa Clara, CA, USA), with built-in crossover, evaluating potential synergistic effects of the two therapies.
Unique features
The safety profile of the device is quite encouraging, especially given the high-risk population of patients who were treated in the studies. In addition, it is a simple and quick procedure, with coronary sinus access being the only appreciable learning curve requirement. It does not preclude any subsequent therapies, including cardiac resynchronisation, and transcatheter mitral valve repair or replacement, and there may be synergy between CARILLON and some of these various therapies3,4.
Potential drawbacks
Contrast is required for the procedure, and therefore renal insufficiency may occur in patients with underlying renal insufficiency. It is not yet clear which patients with secondary MR may or may not benefit based upon anatomic features, with the distance of the coronary sinus to the mitral annulus not turning out to be relevant5. A small percentage of patients cannot receive the device due to coronary artery compromise; however, with the recapture feature of the device and the ability to deploy a new device in another location, this incidence has been significantly reduced with greater experience.
Conflict of interest statement
S. Goldberg is a consultant for and had stock options in Cardiac Dimensions, Inc. J. Lipiecki is a consultant and a proctor for Cardiac Dimensions, Inc. H. Sievert has a consulting relationship with and is a speaker for Cardiac Dimensions, Inc.
Online data supplement
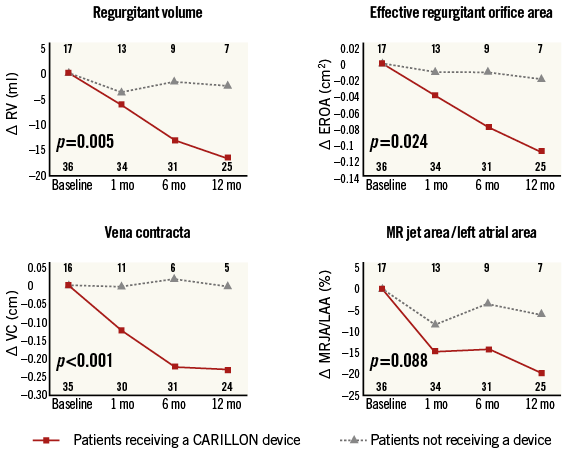
Online Figure 1. Quantitative echo assessments of mitral regurgitation from baseline to one year. There is significant improvement in MR in patients receiving a CARILLON device compared to baseline, and compared to patients not receiving a device, and the MR improves over the one-year period of follow-up2.
Moving image 1. Animation of the CARILLON implant procedure, including release of the device.
Moving image 2. Animation of the CARILLON recapturing procedure.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Animation of the CARILLON implant procedure, including release of the device.
Moving image 2. Animation of the CARILLON recapturing procedure.

