Device description
Name and manufacturer: Accucinch System®; Guided Delivery Systems, Santa Clara, CA, USA.
Approval status: Clinical approval has not yet been granted with safety and efficacy trials ongoing.
Platform: Implanted components: 12-16 nitinol anchors, 6-7 nitinol force distribution members, ultra-high molecular weight polyethylene cinch cable, nitinol locking mechanism.
Delivery components: guide catheter, modular guide tunnel (MGT), anchor delivery catheters, cinch and lock catheter, cut catheter.
Specific design: The system includes 12 to 16 anchors deployed in the subannular left ventricular myocardium. Proximal and distal anchors are interspaced with nitinol force distribution members to disperse the applied tension evenly. Tension is imparted by the cinch cable joining each of the deployed anchors. Additional force distribution members may be placed.
Delivery/approach system: 18 Fr femoral artery sheath.
Device sizes: The guide catheter is 18 Fr with a number of distal tip configurations to accommodate various anatomies, which, together with distal tip deflection, facilitates apposition in the subvalvular space. The number of anchors and the implant length can be individualised based on ventricular size and myocardial thickness on pre-procedural computed tomographic imaging.
Procedural details
The Accucinch device is designed for treatment of significant functional mitral regurgitation (FMR) through placement of appropriately spaced anchors in an arc around the basal left ventricle subannular space. Cinching these anchors reduces the basal left ventricle and mitral annular dimensions (Moving image 1).
The guide catheter is delivered from the femoral artery, retrogradely across the aortic valve with the guide tip shape chosen to sit in the anterior aspect of the subannular space. Deflection of the catheter tip can be used to improve apposition with the endocardial surface. The subannular space is then traversed with a navigation catheter and guidewire. The MGT is then positioned over the guidewire to lie in the subannular space (Figure 1A). The MGT has pre-cut windows on the outer aspect which lie in contact with the left ventricular myocardium. An inner tunnel with a single window can then be gradually withdrawn to align with each of the outer windows and facilitate anchor delivery at appropriately spaced intervals.
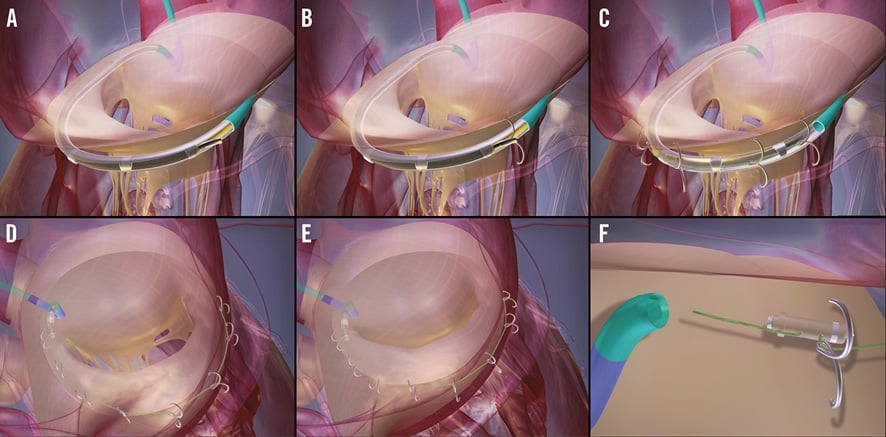
Figure 1. Delivery of the Accucinch System. The subannular space is accessed using the guide catheter and the MGT positioned over a guidewire (A). The inner and outer MGT windows are aligned and anchors delivered (B). The inner MGT window is progressively withdrawn and subsequent anchors delivered (C) until the complete number of anchors has been delivered, joined by the cinch cable (D). The cinch cable is then progressively tightened to reduce the basal left ventricular and mitral annulus dimensions (E). The cinch cable is then locked to maintain the tension and cut (F) prior to removal of the guide catheter and MGT.
The anchors are delivered via anchor delivery catheters. The delivery catheter is manually pushed through the MGT to exit through the aligned windows and advanced 4-5 mm into the left ventricular myocardium (Figure 1B, Figure 1C). The anchor is then deployed with the self-expanding nitinol arms anchoring into the myocardium. The cinch cable is attached to the initial anchor and then fed through subsequent anchors prior to delivery. Anchors are delivered from commissure to commissure through an arc of approximately 220 degrees beneath the posterior leaflet of the mitral valve in the ventricular free wall (Figure 1D).
Force distribution members are spaced between the proximal and distal four anchors to allow even distribution of the applied load and prevent migration of these anchors.
Once the predetermined number of anchors has been deployed, tension is applied to the cinch cable using the cinch and lock catheter (Figure 1E). When an adequate amount of tension has been applied and basal ventricular dimension reduced, the same catheter is used to place a nitinol lock, which maintains the tension within the system. A separate cut catheter is then utilised to cut the cinch cable prior to removal of the MGT and guide catheter (Figure 1F).
Clinical data
The Accucinch device was first implanted in five subjects during an open surgical procedure to demonstrate the feasibility of device delivery and efficacy of the completed implant. A further 17 patients were subsequently treated percutaneously prior to a significant device iteration.
Ongoing studies
The current device iteration is at present undergoing evaluation in the LV Recover and LV Recover SA trials, which are enrolling patients in Australia and Colombia, respectively. Studies in the US Early Feasibility Program and in the EU are planned for 2016.
Unique features
The Accucinch device is unique in its approach of basal left ventricular ventriculoplasty to reduce mitral annulus and basal LV dimensions, as well as to release leaflet tethering. As FMR is often the result of left ventricular dilation, this tackles the problem at the ventricular level, where it originates. It also provides for smaller left ventricular basal dimensions.
Potential improvements
REDUCED CATHETER NUMBERS
Currently a separate anchor delivery catheter is required for placement of each anchor. Development of a multi-delivery catheter or more widely spaced anchors with a reloadable system would reduce the need for catheter exchanges and reduce procedural time.
IMPLANTATION DEPTH GUIDE
Judgement of anchor depth currently relies on fluoroscopic imaging. The addition of a depth guide to the anchor delivery catheter would reduce the risk of inadequate tissue penetration or ventricular perforation. This is in development with successful preclinical work completed.
REDUCTION IN PROCEDURAL STEPS
Easier removal of the MGT and guide catheter at the end of the procedure would reduce procedure times and preserve implant integrity. This is anticipated in the next clinical series.
Acknowledgements
The authors would like to thank Dr Niel Starksen for his constructive review of the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Online data supplement
Moving image 1. Fluoroscopic imaging of a standard Accucinch deployment. The Accucinch deployment involves: placement of a shaped guide catheter retrogradely across the aortic valve and into the subannular space, wiring of the subannular space, placement of a modular guide tunnel over the guidewire, deployment of 12-14 anchors with force distribution members between proximal and distal anchors, removal of the modular guide tunnel, cinching of the cinch cable and placement of a lock to maintain the applied tension within the system, and cutting of the cinch cable to leave the completed implant prior to removal of the guide catheter.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Fluoroscopic imaging of a standard Accucinch deployment. The Accucinch deployment involves: placement of a shaped guide catheter retrogradely across the aortic valve and into the subannular space, wiring of the subannular space, placement of a modular guide tunnel over the guidewire, deployment of 12-14 anchors with force distribution members between proximal and distal anchors, removal of the modular guide tunnel, cinching of the cinch cable and placement of a lock to maintain the applied tension within the system, and cutting of the cinch cable to leave the completed implant prior to removal of the guide catheter.

