
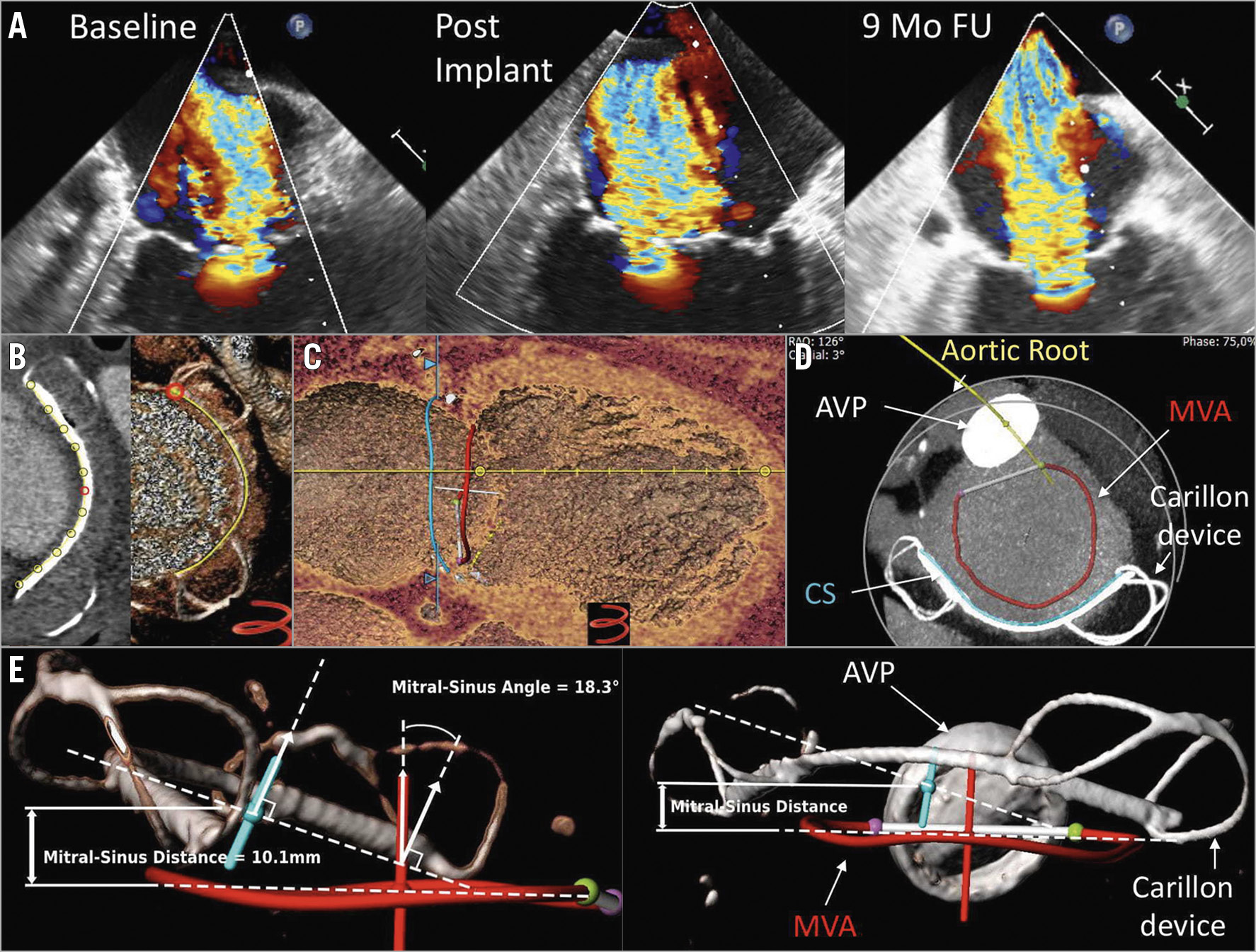
Figure 1. Imaging following unsuccessful Carillon device implantation. A) Transoesophageal echocardiography (TEE) at baseline, post Carillon implantation (post implant) and at nine-month follow-up (9-mo FU). B) Tracking the Carillon device (yellow line). C) MVA to Carillon device planes showing localisation of the device superior to the MVA. D) Aortic root (yellow line); AVP: aortic valve prosthesis; CS: coronary sinus (blue line); MVA: mitral valve annulus (red line). E) Left: topographical model indicating a Carillon device to MVA distance of 10.1 mm and a Carillon device to MVA angle of 18.3°. Right: AVP: aortic valve prosthesis, Carillon device plane vector (blue line); MVA: mitral valve annulus (red line).
Coronary sinus (CS) to mitral valve annulus (MVA) distance and angle might predict echocardiographic response to Carillon device implantation (distance <6.9 mm and angle <14.2°)1. We present the case of a 73-year-old male with aortic valve replacement 10 years before, who underwent implantation of the Carillon Mitral Contour System® (Cardiac Dimensions Inc., Kirkland, WA, USA) due to functional mitral valve regurgitation (FMR). The intervention did not result in an improvement of echocardiographic FMR parameters up to nine months (effective regurgitant orifice area [EROA] at baseline: 0.21 cm2, post implant: 0.22 cm2 and at 9-month follow-up: 0.29 cm2) (Figure 1A). The patient also underwent computed tomography (CT) angiography for coronary artery disease evaluation at nine-month follow-up. We used this CT scan for 3D reconstructions of the Carillon device and MVA topography (3mensio Structural Heart software; Pie Medical Imaging, Maastricht, the Netherlands) (Figure 1B, Figure 1C, Figure 1D). We found a distance of 10.1 mm and an angle of 18.3° between the Carillon device and the MVA planes (Figure 1E), both values implying an unfavourable echocardiographic outcome after Carillon implantation1. Unfortunately, we have no CT scan prior to the Carillon device implantation to investigate the topographical changes caused by the Carillon device.
Conflict of interest statement
H. Degen is a consultant for Biotronik and Cardiac Dimensions. M. Haude has received institutional grants from Abbott and Biotronik, and honoraria from Biotronik, Cardiac Dimensions, OrbusNeich and Philips. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

