
We thank Kuzemczak and colleague1 for their valuable comments on our manuscript “CT-Angiographic imaging of the Carillon device topography after unsuccessful treatment of FMR”2. Our report in the section “Interventional Flashlight” is based on our recently published short report in EuroIntervention, which investigated the relationship between coronary sinus (CS) and mitral valve annulus (MVA) topography and the echocardiographic outcome on functional mitral valve regurgitation (FMR)3. In this analysis we used CT-angiographic imaging prior to the Carillon device implantation for prediction of the echocardiographic outcome (FMR parameters). Our results showed that shorter distance and lower angulation of the CS plane to the MVA plane had an impact on procedure success up to three-month follow-up. Therefore, a distance of <6.9 mm and an angle of <14.2° predicted a reduction of FMR after Carillon device implantation. Despite the small number of patients in this short report, we found a good discrimination between responders and non-responders with no substantial overlap. This implied a potential benefit of a CT-angiographic screening to predict acute and long-term response of FMR after Carillon device implantation.
We agree that implantation of the Carillon device substantially modifies the CS course. To date, no study has investigated how the CS-based mitral annuloplasty changes CS to MVA topography. Since clinical trials with pre- and post-CT scans are difficult to conduct, we presented for the first time CT imaging of the Carillon topography post implantation in a patient without a response on echocardiographic FMR parameters. Unfortunately we have no CT scan prior to the Carillon device implantation to investigate topographical changes due to the CS-based mitral annuloplasty, which would help to gain further insight into the mechanism of device failure. However, we found a distance of 10.1 mm and an angle of 18.3° between the Carillon device and the MVA planes, both values being in the predictive range of an unfavourable echocardiographic outcome after Carillon implantation following our previously published case report. Furthermore, applying tension on the device will not increase the distance of the CS to the MVA, whereas it remains unclear how the angle will change post implantation. Our report will therefore encourage further research in this field, since it visualises the topographical problems of the CS-based indirect mitral valve annuloplasty.
In the AMADEUS trial sub-analysis, no difference of the CS position relative to the MVA was found in patients with or without acute reduction of FMR based on multislice CT4. However, linear distances from the edge of the MVA to the centre of the vein lumen were exclusively measured in long-axis views and the distance to the MVA is highly variable over the course of the CS. Therefore, single measurements are not reliable to describe the topography (Figure 1). We therefore used three-dimensional reconstructions generating an MVA and CS plane (3mensio Structural Heart; Pie Medical Imaging BV, Maastricht, the Netherlands) to determine the distance more precisely. However, our previously published study is hypothesis-generating only. More clinical trials are needed prior to routine usage.
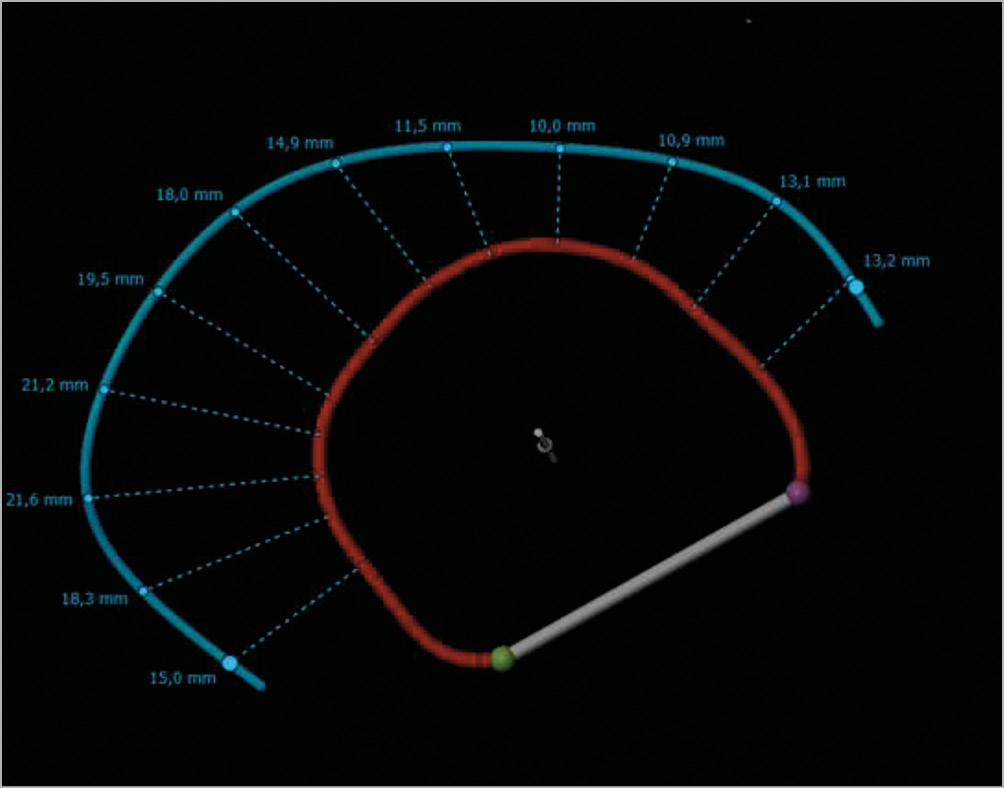
Figure 1. Variable distances to mitral valve annulus over the course of the coronary sinus. Blue line: coronary sinus; red line: mitral valve annulus. Distances as indicated.
We agree with Kuzemczak et al that echocardiographic screening prior to Carillon device implantation is important for procedural outcome. We routinely determine the tenting area prior to device implantation and of course CT screening strategies cannot replace this valuable clinical algorithm. Nevertheless, we reported the first evidence that CT screening prior to mitral valve annuloplasty using a CS-based device could help to improve clinical outcome, but of course future studies need to validate CT screening algorithms in a prospective trial.
Conflict of interest statement
H. Degen is a consultant for Biotronik and Cardiac Dimensions. M. Haude is a consultant for Biotronik, OrbusNeich and Abbott, and received grant support from Biotronik, OrbusNeich, Abbott, Medtronic and Cardiac Dimensions. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

