Description - technical specifications
THE MEDTRONIC ENGAGER VALVE PROSTHESIS
The EngagerTM aortic valve bioprosthesis (Medtronic, Inc., Minneapolis, MN, USA). consists of a self-expanding nitinol frame and a biological heart valve. The stents consist of a main frame and a support frame. The control arms of the support frame are designed to be placed into the sinus of the aortic root to achieve an anatomically correct position in a defined height of implantation to minimise the risk of coronary obstruction. The main frame is sewn to a polyester sleeve. Three leaflets, cut from tissue-fixated bovine pericardium are mounted on the stent (Figure 1). The valve design is intended to avoid paravalvular leaks. The prosthesis is available in two sizes (23 mm and 26 mm) covering annulus diameters from 21 to 27 mm.

Figure 1. The Medtronic Engager transcatheter aortic valve implantation prosthesis. (©Medtronic, Inc. Printed with permission).
THE MEDTRONIC ENGAGER DELIVERY SYSTEM
Implantation is performed transapically with an over-the-wire delivery system (compatible with 0.035” wires), comprising an introducer and a flexible delivery catheter which form one integral unit (Figure 2). The delivery system is composed of a 29 Fr (inner diameter) introducer and a flexible delivery catheter with a 13 Fr shaft. The introducer stays in the apex while the shaft with the completely covered valve prosthesis is advanced through the aortic valve. The delivery system allows for a three-step release of the prosthesis by first releasing the control arms and releasing the commissural posts after unlocking a safety button when a correct positioning has been achieved. The last step is the full release of the self-expanding valve. Repositioning can be performed until unlocking the safety button and releasing the commissural posts.
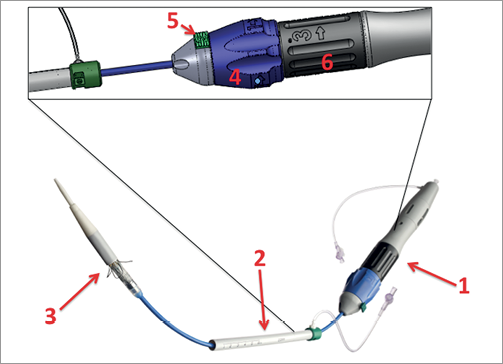
Figure 2. Medtronic Engager delivery system. 1) Handle of the device. 2) Introducer. 3) Delivery catheter. 4) Blue knob to release control arms. 5) Safety button. 6) Black knob for final release and deployment of the valve. (©Medtronic, Inc. Printed with permission).
IMPLANTATION PROCEDURE
Patients are prepared in a standard fashion for a transapical TAVI procedure, with cardiopulmonary bypass on stand-by and a guidewire placed over the femoral vein in the right atrium as a safety precaution.
BALLOON VALVULOPLASTY
Balloon valvuloplasty is recommended before every Medtronic Engager prosthesis implantation.
IMPLANTATION OF THE PROSTHESIS
The delivery system with the mounted Engager valve is then inserted over the guidewire and, with the introducer held fixed at the desired depth, the delivery catheter is advanced across the aortic valve under fluoroscopic guidance. Commissural alignment is performed using the rotational positioning technique. For anatomical positioning, C-arm alignment is optimal with a three-cusp view with the native valve commissure between the non- and left coronary cusps in a posterior position in the middle of the right coronary cusp. This view is normally achieved with a slight LAO/RAO C-arm angulation or direct AP angulation (Figure 3).
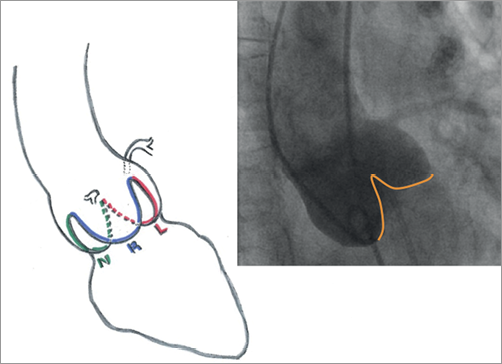
Figure 3. Implanter’s view (AP or slightly RAO/LAO), for the Medtronic Engager bioprosthesis. The yellow line in the angiography shows the native commissure defined as the intercusp triangle.
ROTATIONAL POSITIONING
For anatomical alignment of the valve, a small window at the top of the commissural posts is used that should be aligned with the native commissure between the left- and non-coronary cusps. By rotating the introducer, the direction of the movement of the window indicates if it is in a posterior or an anterior position. When movement towards the non-coronary cusp is seen by rotating the handle clockwise, the post is in the aimed posterior position (Moving image 1).
As a first step of the implantation of the prosthesis, the support arms must be placed into the sinuses of the aortic valve. The support arms are gradually exposed by control of a blue rotating knob at the handle. After exposure, the delivery catheter is withdrawn slowly under fluoroscopic and tactile control until the support arms are positioned in the sinus which is recognised as resistance (Figure 4). Correct subcoronary positioning is verified by aortic root angiography. Repositioning (if necessary with recapture of the support arms) could be performed at this stage.
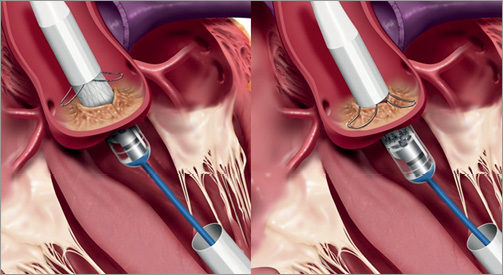
Figure 4. First step of implantation. Left picture: gradually exposed control arms after rotational positioning. Right picture: positioning of the control arms in the sinus under tactile and angiographic feedback. (©Medtronic, Inc. Printed with permission).
At this point, a safety button, that prevents early deployment of the prosthesis before the aimed position is achieved, has to be unlocked. The commissural posts are uncovered by further rotating the blue knob. For final self-expandable deployment the second (black) knob at the handle has to be rotated (Figure 5). At this stage it is essential to hold the support arms engaged against the valve. The final deployment should be performed under rapid ventricular pacing. With the last turn of the knob, the device is released into its final position.
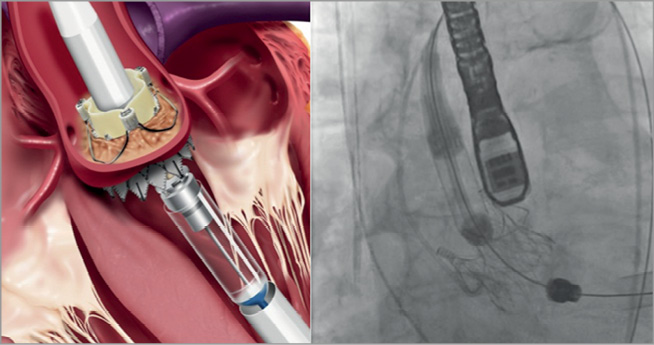
Figure 5. Final release of the valve prosthesis. (©Medtronic, Inc. Printed with permission).
The delivery system is reconnected with the introducer tube under fluoroscopic control, and then the whole system including the guidewire is removed. The apex is closed with the purse-string sutures. Valve position and function should be assessed immediately using angiographic and echocardiographic imaging as well as by simultaneous recording of the left ventricular and aortic pressure curves. The intercostal incision is closed in a standard fashion.
Postoperative device-specific medical therapy of aspirin 100 mg daily indefinitely and clopidogrel 75 mg daily for at least three months is recommended.
CLINICAL RESULT
A feasibility study included ten patients with a mean age of 82.5 years and a mean logistic EuroSCORE of 24.6% who were successfully implanted with anatomically correct positioning1. The invasively measured gradient was 7.1 mmHg after implantation. In nine of the implanted patients, there was no or only trivial paravalvular leak. In one patient, aortic regurgitation grade 1-2 was observed. One implanted patient (logistic EuroSCORE 48.9%) expired at postoperative day 23 in multiorgan failure. At 30-day follow-up mean gradient measured by TTE was 16.2 mmHg and no more than mild trans- or paravalvular leakage was present. Two patients required permanent pacemaker implantation.
This feasibility study was followed by a European pivotal trial including 125 patients. An interim analysis after the 30-day follow-up in 61 patients was performed for CE mark submission2. Mean age was 81.9 years and the mean logistic EuroSCORE was 18.9%. Overall device success, defined by modified VARC criteria, was achieved in 94.3%. All cause-mortality was 9.9% at 30 days. One patient had a stroke, one patient suffered from a myocardial infarction, one patient had a life-threatening bleeding, five patients had major apical access-site complications, and 16 patients had indications for new pacemaker implantation. None of the patients had more than trace paravalvular leakage at 30-day follow-up. The CE mark was received in February 2013.
Discussion
The Medtronic Engager transapical transcatheter system has control arms that are intended to facilitate placement in the correct position to prevent severe complications like coronary obstruction by a TAVI prosthesis implanted too high. Furthermore, an implantation height of a TAVI prosthesis that is too low seems to be of importance with regard to new conduction disturbances. Low implantation was associated with an increase in new left bundle branch block and total heart block with the Edwards SAPIENTM prosthesis (Edwards Lifesciences, Irvine, CA, USA) in a prospective study with 89 patients, of whom 5.1% needed a new pacemaker implantation. All bioprostheses in these patients were implanted significantly lower than in the comparison group3. For the CoreValve® bioprosthesis (Medtronic), a self-expandable prosthesis like the Engager bioprosthesis, the rate of new pacemaker implantations is described as around 30 to 40% in most centres, but up to 67% has been reported4. New conduction disturbances also have an impact on outcome. Patients without new left bundle branch block or new pacemaker implantation have a better recovery of the left ventricular function after TAVI5.
The design of the lower skirt is intended to prevent paravalvular leakage. Current literature provides evidence that the rate of moderate to severe paravalvular leakage is much higher after TAVI procedures than after surgical aortic valve replacement (6.9% vs. 0.9%) and that even mild paravalvular leakage is associated with an increase of late mortality6. No moderate or severe paravalvular or transvalvular regurgitation has been observed during 30-day1 and one-year follow-up (unpublished data) with the Engager bioprosthesis during the feasibility study and at 30-day follow-up in the interim analysis of the European pivotal trial2. This might indicate a potential advantage of the design of the bioprosthesis’ lower skirt with regard to the reduction of paravalvular leakage. Both paravalvular leakage and the need for new pacemaker implantation are of particular importance when discussing TAVI as therapy for younger patients or patients with a lower risk profile, given the excellent outcomes after surgical aortic valve replacement even in octogenarians7.
Actually, there is no evidence that anatomical positioning of transcatheter aortic valve bioprostheses has benefits, but more companies have developed valves with similar features like the JenaValve (JenaValve Technology GmbH, Munich, Germany)8 and the Symetis Acurate TATM (Symetis, Ecublens, Switzerland)9 devices, which are also designed for the transapical approach, indicating that this approach is assumed to be favourable by different companies. Further studies comparing these second-generation devices with first-generation devices have to be conducted to prove the hypothetical advantages.
There are some limitations of the Medtronic Engager bioprosthesis. At the moment it is only available for the transapical approach and, after complete release of the prosthesis, there is no possibility of retrieval of the valve.
In conclusion, the Medtronic Engager transapical transcatheter valve system is a second-generation TAVI system made for transapical access. The device has features to facilitate correct positioning and is designed to reduce paravalvular leakage. Three multicentre trials have been conducted so far with promising results. CE-mark approval followed in 2013.
Conflict of interest statement
D. Holzhey, S. Bleiziffer and H. Treede are proctors for Medtronic. H. Treede and V. Falk are advisors for Medtronic, V. Falk is consultant for Valtech Cardio, receives lecture fees from Medtronic and Edwards Lifesciences and holds a research grant from Philips. S.H. Sündermann has no conflicts of interest to declare.
Online data supplement
Moving image 1. Rotational positioning. The angiography shows the delivery system with the mounted, crimped valve prosthesis placed in the native aortic valve. The delivery system is rotated clockwise and the window above the commissural post rotates to the left side indicating the correct posterior position. The step of rotational positioning is shown schematically and as animation in the right upper inlay picture.

