Abstract
Aims: To present the long-term results of prednisone-treated patients enrolled in the IMPRESS studies. Such studies demonstrated the efficacy of a short course of immunosuppression with oral prednisone after percutaneous coronary intervention (PCI) with bare metal stent (BMS) implantation compared to BMS alone at one year.
Methods and results: Eighty-four non-diabetic patients with elevated C-reactive protein after PCI treated with BMS and prednisone, were followed clinically for a minimum of five years. Event-free survival was defined as freedom from death, myocardial infarction, and need for target vessel revascularisation. Event-free survival rate at a mean of 6.5±1.4 years was significantly better in prednisone-treated patients of the IMPRESS and IMPRESS-2/MVD respectively compared to their original control arms: 87.8 versus 47.6%, relative risk: 7.9; 95%CI: 2.6–24.1, p<0.0001, log-rank=13.06, p=0.0003; and 93 versus 60.5%, relative risk: 8.7; 95%CI: 2.3–32.7, p=0.0004, log-rank=13,18, p=0.0003, respectively. The event-free survival was 54.1% in controls and 90.5% in the prednisone group; relative risk: 8.1; 95%CI: 3.5–18.7, p<0.0001, log-rank= 26.33, p<0.0001.
Conclusions: The clinical benefits of oral treatment with prednisone after conventional PCI in non-diabetic patients with evidence of systemic inflammation after stenting are maintained at long-term follow-up, either in patients with single or multivessel coronary artery disease.
Introduction
The importance of inflammation in the mechanisms of restenosis after percutaneous coronary interventions (PCI) is well known1-2. Accordingly, sustained immunosuppressive doses of oral prednisone proved the efficacy of glucocorticosteroids to prevent restenosis after implantation of bare metal stents (BMS) in the randomised “Immunosuppressive therapy for the prevention of restenosis after coronary artery stent implantation” (IMPRESS) study3 that treated patients with single-vessel coronary artery disease and in the IMPRESS-2/MVD registry, that included patients treated with multivessel PCI4. Of note, based on the study protocols, all prednisone-treated patients enrolled in these two previous studies exhibited elevated C-reactive protein (CRP) levels, thus implying that the efficacy of the prednisone treatment may be elicited in patients with enhanced inflammatory response following coronary stent implantation.
Undesirable side-effects of steroid-based therapies however, may reduce the net benefit of this treatment and prevent broad acceptance. The mechanisms involved in the possible development of side effects of this treatment, as well as their incidence, and the preventive measures to avoid or to minimise their manifestations, have been recently disclosed in detail5.
The aim of this study was to investigate whether the benefit of oral treatment with prednisone after PCI is sustained at long-term, either in patients with single or with multivessel coronary artery disease.
Methods
Patients
We conducted a prospective analysis of the long-term clinical outcome of the prednisone-treated patients enrolled in the IMPRESS (n=41) and IMPRESS-2/MVD (n= 43) studies3-4. With this aim, the two prednisone-treated arms were analysed separately and pooled together. Furthermore, we reported the comparisons of long-term outcomes between prednisone-treated patients and their controls in each of the two IMPRESS studies.
Briefly, the first IMPRESS study was a randomised single-blinded study performed on 83 patients undergoing single BMS implantation, starting the oral treatment after 48-72 hours of PCI and with evidence of elevated CRP levels, i.e. >0.5 mg/dl analysed by immuno-turbidimetric method. Patients received either placebo (n=42) or oral prednisone (n=41) according to the following treatment scheme: oral prednisone at a dose of 1 mg/kg for 10 days, 0.5 mg/kg for 20 days and 0.25 mg/kg for 15 days3. This dosage is in part derived from the immuno-supressive protocol applied after heart transplantation6. The IMPRESS 2/MVD study included 86 patients (43 prednisone-treated patients and 43 controls) with diffuse multivessel coronary artery disease treated with multiple PCI. Patients without contraindication to prednisone and high CRP levels 48-72 hours after PCI received the same steroid treatment used in the first IMPRESS trial4.
All patients were pretreated with aspirin and a thienopyridine, either ticlopidine (250 mg twice daily for 72 hours) or clopidogrel (300 mg loading at least 6 hours before PCI); the thienopyridine was recommended for one month, and aspirin indefinitely. Contraindications to enter the studies were: implantation of drug-eluting stents (DES), life expectancy <24 months, contraindication to the use of aspirin or thienopyridines, and contraindications to steroid use including: diabetes, recent Q-wave myocardial infarction (<4 weeks), active peptic ulcer, active infectious disease, and uncontrolled severe hypertension.
Follow-up and endpoints
All patients were controlled clinically 30 days after the procedure to assess the correct and complete assumption of the medical therapy and the eventual occurrence of side effects. All patients underwent a provocative stress test within six months after PCI, and a subsequent out-patient clinic control. Twelve months after the index procedure, all patients were controlled in the out-patient clinics by assigned independent clinicians to verify the occurrence of any major adverse cardiac event considered in the primary endpoint of the studies: death (from any cause), myocardial infarction after discharge (defined as the rise and fall of creatine-kinase MB and new ischaemic electrocardiographic changes), and recurrence of symptoms or ischaemia requiring new revascularisation of the treated vessel. Stent thrombosis was defined as either acute myocardial ischaemia with angiographic evidence of intracoronary thrombus, or unexplained cardiac death due to a possible or presumed thrombosis causing MI that involves the target vessel territory irrespective of the time after the index procedure. Stent thrombosis occurring after one month and before one year of implantation were defined as “late”, and “very late” thereafter.
After the first year of follow-up, patients have been contacted periodically either by telephone or by means of outpatient clinic controls, to update their event-free survival follow-up up to a minimum of five years since the index procedure. The analysis of events has been performed on a hierarchical basis. The events analysed as indicators of clinical efficacy at long-term were the following: death of any cause, myocardial infarction and recurrence of angina or ischaemia needing repeated revascularisation. The occurrence of clinical events that may be related to the long-term safety of the steroid therapy were also monitored, in particular: gastric bleeding, spontaneous bone fractures due to osteoporosis, ischaemic vascular events due to the worsening of arterial disease in the peripheral or coronary circulation, and the development of diabetes during the follow-up period.
The use of prednisone to prevent restenosis after PCI had been previously approved by the Ethical Committees of our hospitals and all patients enrolled gave their informed consent according to ethical regulations.
Statistical analysis
Long-term outcome was analysed per protocol. Continuous data are expressed as means and standard deviations; discrete variables are given as absolute values and percentages. The Student’s t-test was used to compare differences between continuous variables. The chi-square statistic with Yates’ correction, or Fisher’s exact test when appropriate, were used to test associations of categorical data. Survival analysis was performed by Kaplan-Meier method. Differences in survival parameters were assessed for significance by means of the log-rank test. All tests were two-sided. A probability value of less than or equal to 5% was considered significant.
Results
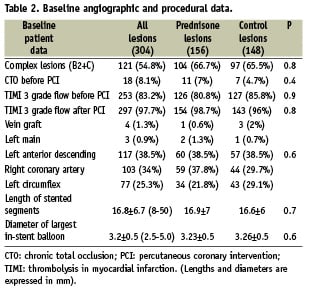
Baseline characteristics of all the prednisone-treated patients and controls are disclosed in Tables 1 and 2. Details of patients enrolled in each IMPRESS study, and the comparison of 1-year clinical outcome between prednisone-treated patients and their controls have been previously published3,4.
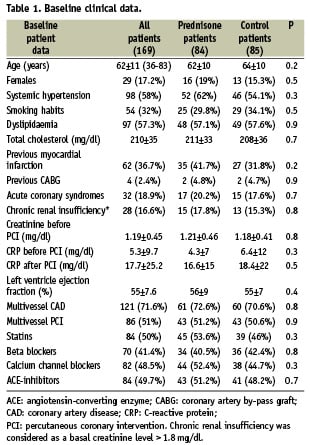
Side effects of the steroid therapy were detected in nine out of 84 prednisone-treated patients (11%), namely gastric pain in four cases, hypertension requiring temporary upgrading of the usual treatment in three, and reversible fluid retention and weight gain in one case.
Clinical follow-up at one year of the IMPRESS and IMPRESS-2/MVD studies was obtained in all prednisone-treated patients and is reported in the Table 3. The overall 12-month event-free survival rate in both studies was 93%.
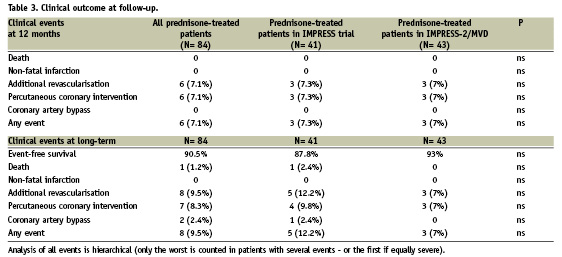
All the 41 prednisone-treated patients enrolled in the first IMPRESS study and the 43 prednisone-treated patients enrolled in the IMPRESS-2/MVD study were evaluated during a mean follow-up period of 6.5±1.4 years (2867±278 days and 1951±227 days respectively). Among prednisone-treated patients, the cumulative incidence of clinical events since the index procedure was 12.2% and 7%, in the IMPRESS and IMPRESS-2/MVD, respectively (Table 3). In particular, the events occurring after 12 months of the index procedure were the following: one patient died due to refractory heart failure after 4.5 years; the patient presented a severe three vessel disease and underwent a re-PCI on one vessel at 210 days. All other new events were repeated target vessel revascularisation due to recurrence of angina. All new revascularisations included a previously treated lesion.
Compared to their original control arms, both prednisone-treated arms exhibited better event-free survival rates (87.8 versus 47.6%, relative risk: 7.9; 95%CI: 2.6 – 24.1, p<0.0001, log-rank=13.06, p=0.0003; and 93 versus 60.5%, relative risk: 8.7; 95%CI: 2.3 – 32.7, p=0.0004, log-rank=13,18 p=0.0003, respectively) (Figure 1, a-b). The event-free survival was therefore 54.1% in controls and 90.5% in the prednisone group pooled together; (relative risk: 8.1; 95%CI: 3.5 – 18.7, p<0.0001, log-rank= 26.33, p<0.0001).
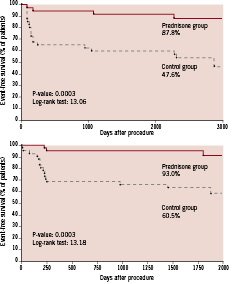
Figure 1, a-b. Event-free survival curves (death, non-fatal myocardial infarction and repeated target vessel revascularisation due to recurrence of ischaemia) at long-term follow-up in patients with single-vessel disease in the first IMPRESS study (a) and in patients with multivessel disease enrolled in the IMPRESS 2/MVD study (b).
Regarding the assessment of safety at long-term in the prednisone-treated patients, no patient had gastric bleeding or spontaneous bone fracture; a peripheral vessel revascularisation procedure was performed in two patients and a new onset of diabetes after the index procedure was diagnosed in three patients (in all cases after the first year from treatment). Of note, no acute ischaemic event (sudden death, acute myocardial infarction, late or very late stent thrombosis) occurred, despite early discontinuation of dual antiplatelet therapy (one month) in most cases.
Prednisone treatment equalised the long-term risk of encountering clinical events according to the post-PCI CRP levels analysed by percentiles (7.1%, 13%, 8.7%, p=0.8), while among controls, higher CRP levels correlated significantly with the occurrence of MACE at the long-term, (24%, 62%, 48%, p=0.001).
Discussion
The main finding of this study is that clinical benefits of oral treatment with prednisone after conventional PCI in non-diabetic patients with elevated CRP levels after coronary stenting are maintained at long-term, either in those with single or with multivessel coronary disease. Of note, the event-free survival rate of prednisone-treated patients is similar to that observed among DES-treated patients enrolled in large multicentre trials at a comparable long-term follow-up7, while the long-term outcome of control patients treated with BMS only is very similar to that observed by others8-9. However, because of the exclusion of diabetic patients in the IMPRESS studies, a more favourable, long-term outcome than that observed in the present study would have been expected. The high incidence of MVD (> 70%), the low use of statins (< 50%), and in particular, the selection of patients with evidence of persistently high levels of CRP after PCI (100%) could have played a determinant role on their prognosis10.
Another relevant finding of this study is the very low incidence of clinical events that might be somehow related to the long-term safety of the steroid therapy. In particular, two patients (2.4%) underwent a peripheral revascularisation procedure and three (3.6%) developed diabetes during the long follow-up period. Thus, although the possible detrimental actions that a steroid treatment may cause in the cardiovascular system at the long-term do need an accurate prospective assessment11-12, our results suggest that a short-term steroid exposure, such as that tested in the IMPRESS trials (45 days) is unlikely to induce permanent vascular damage.
No acute ischaemic events (sudden death, acute myocardial infarction, late or very late stent thrombosis) occurred in our patient population, despite early discontinuation of dual antiplatelet therapy in most cases. Recent experimental results obtained by our group in the animal model with BMS implantation, followed by administration of oral prednisone according to the same therapeutic scheme used in humans, showed a significant reduction of the neointimal proliferation compared to BMS alone, and a complete re-endothelialisation of the stent struts, as compared to the commercially available paclitaxel-eluting stent13. Such findings support the favourable healing profile of this therapy that may translate into safe, long-lasting, clinical results, without the need for prolonged dual antiplatelet therapy.
These results cannot be extrapolated to all patients undergoing PCI, but rather to those without contraindications to corticosteroid treatment and with evidence of an enhanced inflammatory response after coronary stenting, i.e., about 20% of coronary patients referred to our centres for PCI3,4. Furthermore, we have previously demonstrated that lower doses of prednisone are less effective in preventing restenosis since the treatment is dose-sensitive14, but unlike other experiences with systemic drug administration, prednisone does not need to be started before the procedure. Nonetheless, it is likely that the specific setting of patients with persistently high CRP plasma levels after the procedure are those who might benefit most from the anti-inflammatory effects of prednisone, a population at risk for a worse long-term prognosis after PCI10.
The finding that prednisone treatment equalised the long-term risk of encountering clinical events according to the post-PCI CRP plasma levels together with an absolute very low incidence of events, either in patients with single or with multivessel disease, further supports the use of a short-course oral prednisone treatment after BMS implantation. These findings also suggest that the systemic efficacy of the treatment may be in part independent from conventional characteristics known to modulate the long-term outcome of PCI, a similar effect to that observed with the administration of statins. In fact, statins are also capable of reducing CRP levels and have beneficial effects on clinical outcome after PCI15. The favourable effects of statins and steroids, acting through different mechanisms on the pathways of atherosclerosis could be likely additive, and this underlines the importance of addressing atherosclerosis as a systemic disease rather than as a local problem that can be simply solved with a mechanical intervention. Although treatment with statins has been shown to offer benefit to most patients with evidences of atherosclerosis, establishing whether systemic therapy with prednisone serves as an adjunctive therapy only in selected patients with persistent evidence of systemic inflammation after coronary stenting, or if it may be an alternative to DES in larger populations independently of periprocedural CRP measurements has been addressed in a larger randomised trial that included patients irrespective of the CRP levels (NCT00369356).
Limitations
The combined analysis of the two studies (both prednisone-treated and control arms) needs to be interpreted with caution because of some methodological and baseline differences of the two studies. Thus, the present analysis is mainly focused on the prednisone-treated patients only, and the comparisons with controls is just briefly reported in a separate manner. Another shortcoming concerns the applicability of these results, the exclusion of diabetic patients being the main restriction of this treatment.

