Abstract
Aims: To assess the immediate and long-term outcomes of transcatheter closure of ventricular septal defect (VSD) in combination with percutaneous coronary intervention (PCI) in patients with VSD complicating acute myocardial infarction (AMI).
Methods and results: Data were prospectively collected from 35 AMI patients who underwent attempted transcatheter VSD closure and PCI therapy in five high-volume heart centres. All the patients who survived the procedures were followed up by chest x-ray, electrocardiogram and echocardiography. Thirteen patients underwent urgent VSD closure in the acute phase (within two weeks from VSD) while the others underwent elective closure at a median of 23 days from VSD occurrence. The percentage of VSD closure device success was 92.3% (36/39) and procedure success was 91.4% (32/35). The incidence of in-hospital mortality was 14.3% (5/35). At a median of 53 months follow-up, only two patients died at 38 and 41 months, respectively, and other patients’ cardiac function tested by echocardiography improved significantly compared to that evaluated before discharge.
Conclusion: The combination of transcatheter VSD closure and PCI for treating VSD complicating AMI is safe and feasible and is a promising alternative to surgery in patients with anatomically suitable VSD and coronary lesion.
Introduction
Ventricular septal defect (VSD) following acute myocardial infarction (AMI) is a fatal complication which occurred in 1% to 3% of patients with AMI in the era before reperfusion therapy1. Risk factors for VSD include hypertension, advanced age, female gender, diabetes mellitus, the absence of a history of angina or myocardial infarction (MI), and severe coronary stenosis or total occlusion without compensatory collateral circulation2-4. Despite advances in thrombolysis and percutaneous coronary intervention (PCI) therapy and a perceived decline in the incidence of post-infarction VSD, this still occurs in 0.2% of all patients with AMI5-7. To date, post-infarction VSD still carries a poor prognosis8,9. Over 80% of untreated patients die within the first month, and >90% within the first year after VSD following AMI6,10,11. The mortality of patients with cardiogenic shock due to VSD is as high as 67% within 48 hours and 100% within 30 days12. It is well documented that surgery in the early stage is favourable for survival13. However, the mortality rate is approximately 50% with surgery performed within three weeks and as high as 38% when performed three weeks after AMI onset14,15. The incidence of a large residual shunt and re-rupture after surgery reaches up to 10%~20%8,9,16 and among patients who survived the perioperative period the five-year survival rates reported in two studies were only 50% (n=102) and 67% (n=112)17,18. With advances in cardiac interventional techniques and devices, percutaneous intervention has now been developed for many cardiovascular diseases including congenital heart disease19 and complex coronary artery disease20.
Transcatheter VSD closure has become a treatment option for patients with post-infarction VSD5-7, even in the acute setting21. However, clinical experience with transcatheter closure of post-AMI VSD in combination with PCI therapy is limited. We evaluated the long-term safety and efficacy of combined transcatheter VSD closure and PCI in 35 cases of AMI patients with the additional complication of post-infarction VSD from five Chinese heart centres.
Methods
STUDY PATIENTS
AMI patients with the additional complication of post-infarction VSD who underwent attempted transcatheter VSD closure and PCI therapy were enrolled. Data were collected prospectively with uniform case report forms including general demographic and clinical data, patient’s history, laboratory findings, procedure and device details, as well as occurrence of complications and deaths. Inclusion criteria included the presence of a VSD as a result of preceding MI, diagnosed by echocardiogram, and at least one coronary lesion requiring treatment percutaneously. In patients with an occluded culprit vessel, stress echocardiography and/or single-photon emission computed tomography were used to prove the existence of myocardial ischaemia at the physician’s discretion. There were no general clinical or haemodynamic exclusion criteria, but patients with a VSD sizing on echocardiography in excess of the maximum available size of the post-infarction muscular VSD device and left ventricular aneurysm were considered unsuitable for device closure and PCI therapy. Written informed consent was obtained in all cases prior to the procedures. Patients who had undergone a first procedure remained in hospital until the next procedure was performed.
DEVICES
The device used to close the defect was the Amplatzer post-infarct muscular VSD (PIMVSD) occluder (AGA Medical Corporation, Plymouth, MN, USA) or a modified double-disc occluder (Shanghai Shape Memory Alloy Material Co., Ltd, Shanghai, China)22. The coronary stents included bare metal stents (Zeta™; Abbott Vascular, Santa Clara, CA, USA; Bx VELOCITY®; Cordis, Johnson & Johnson, Warren, NJ, USA; Tsunami®; Terumo, Tokyo, Japan; and Accura™; Accura Medizintechnik GmbH, Karben, Germany) and drug-eluting stents (Firebird™; MicroPort Co. Ltd., Shanghai, China; Endeavor®; Medtronic, Minneapolis, MN, USA; and TAXUS® Liberté™; Boston Scientific, Natick, MA, USA).
TIMING OF PROCEDURES AND ANTIPLATELET THERAPY
It was at the physicians’ discretion to determine the timing of the two procedures. Usually, VSD closure was prior to PCI; however, the PCI procedure would be performed first if the patient presented with unstable angina and if coronary angiography showed a culprit lesion in proximal vessels with heavy thrombosis which might cause recurrent MI. In patients who underwent VSD closure first, no antiplatelet drugs were used before the procedure and aspirin was prescribed after VSD closure if there was no residual shunt. For patients with a residual shunt after VSD closure, no antiplatelet drugs were used until one to three days before the PCI procedure and dual antiplatelet therapy administration (DAPT, including aspirin and thienopyridines). In patients who underwent PCI first, DAPT was initiated before PCI, and ceased two days before VSD closure. DAPT was then resumed if there was no residual shunt after VSD closure, otherwise only aspirin was administered.
PROTOCOL OF TRANSCATHETER VSD CLOSURE
The implantation technique was similar to that described by Holzer et al7. Commonly, the right femoral artery and vein were cannulated but, in patients with an apical VSD, the femoral arterial and jugular venous approach was used for arteriovenous guidewire loop creation. The VSD was crossed by using a retrograde approach with a 6 Fr Judkins right coronary catheter which introduced a 0.032 inch×260 cm exchange wire (Terumo, Tokyo, Japan) across the defect into the pulmonary artery or through the right atrium into the superior vena cava. The tip of the exchange wire was snared in the pulmonary artery or superior vena cava by using a 10 mm Amplatz GooseNeck™ snare catheter (Microvena, White Bear Lake, MN, USA) and exteriorised via the right femoral vein or internal jugular vein. Subsequent delivery proceeded similarly to congenital muscular VSD closure. After transthoracic echocardiography and left ventriculography showed ideal placement without or with only a mild (<2 mm) to moderate (<4 mm) residual shunt, the device was released. All patients had a chest x-ray, transthoracic echocardiography, and electrocardiogram within 24 hours following the procedure.
PROTOCOL OF PERCUTANEOUS CORONARY INTERVENTION
The PCI procedures were performed using a standard femoral or radial approach. Reference diameter, minimal luminal diameter, percentage diameter stenosis, and lesion length were measured before and after PCI. The target vessels included both infarction-related coronary arteries and the epicardial non-infarction-related coronary arteries with ≥70% stenosis of lesion. PCI success was defined as restoration of TIMI flow grade 3 with a residual stenosis of <20% in the target lesion after stent implantation, and patient success was defined as procedural success in the target lesion without major complications.
FOLLOW-UP
Before discharge, patients underwent transthoracic echocardiography to confirm correct device position. All patients were followed up by x-ray and echocardiography, and clinically at one, three, six and 12 months after transcatheter closure and thereafter at six-month intervals. The degree of a residual shunt was assessed by measuring the width of the colour jet as it exited through the ventricular septum by echocardiography. It was classified as trivial for a width <1 mm, mild for a width between 1 and 2 mm, moderate for a width between 2 and 4 mm, and large for a width >4 mm.
STATISTICAL ANALYSIS
Continuous parameters are expressed as median with interquartile range (IQR), and categorical variables as percentages. Comparisons of baseline data were performed using the chi-square test or Fisher’s exact test (categorical variables) and the Student’s t-test or Wilcoxon rank-sum test as appropriate (continuous variables). P-values <0.05 were considered statistically significant.
Results
IN-HOSPITAL OUTCOMES
Between April 2001 and September 2011, 232 AMI patients who had the additional complication of post-infarction VSD were assessed for enrolment and, finally, 35 patients were enrolled who then underwent attempted transcatheter VSD closure and PCI therapy. Patient flow is illustrated in Figure 1. Baseline demographic, clinical and angiographic data of those patients are listed in Table 1. Among patients who underwent transcatheter therapy, 18 patients (51.4%) had no previous history of angina or MI at the time of admission. Time from AMI to VSD diagnosis was 4.5 (IQR: 1.5-8.5) days. Echocardiograms showed 39 VSDs in 35 patients (four patients had two separate defects). The left ventricular ejection fraction (LVEF) was 0.45 (IQR: 0.40-0.50) and the left ventricular end-diastolic diameter (LVEDD) was 57 mm (IQR: 52-62). Thirty-two of 35 patients underwent both successful transcatheter VSD closure and PCI therapy, and 30 of them survived until discharge. The total procedural success rate was 91.4% (32/35). The in-hospital survival rates were 85.7% (30/35) for all patients and 93.8% (30/32) for patients who had successful procedures, respectively. Angiographic images of a typical patient who underwent transcatheter therapy are shown in Figure 2.
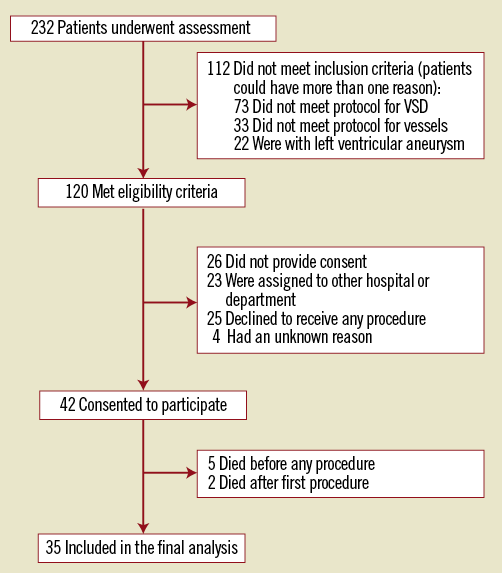
Figure 1. Patient flow.
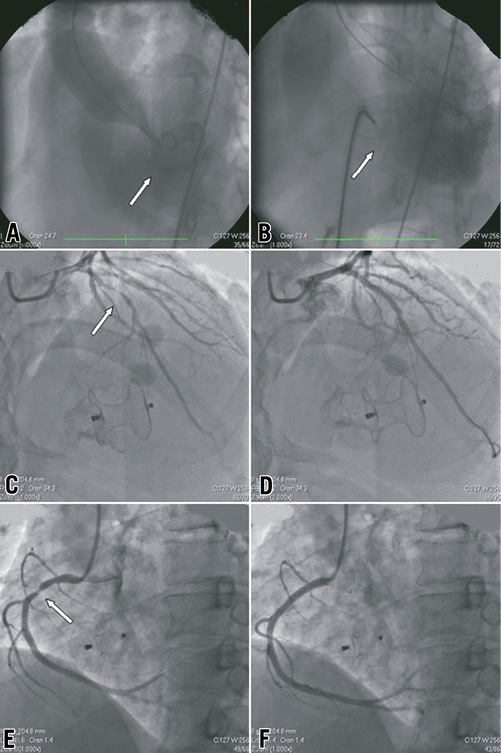
Figure 2. Angiographic images in a 65-year-old male patient. A) LV angiogram delineating the mid muscular VSD (arrow). B) Occluder (arrow) was deployed 3 days after VSD diagnosis, and no residual shunt was found by angiography. C) Coronary angiography showed a 95% stenosis in proximal LAD. D) Angiography after LAD stenting which was undergone 10 days after VSD closure. E) Coronary angiography showed a 90% stenosis in proximal RCA. F) Angiography after RCA stenting in the same PCI procedure as LAD. LAD: left anterior descending coronary artery; LV: left ventricular; RCA: right coronary artery; VSD: ventricular septal defect
Details of the five patients who died in hospital after transcatheter therapy are as follows: patient 1 was a female who had a history of cerebral infarction. Her status was good after successful transcatheter VSD closure and PCI therapy; however, a week later, the patient died of cerebral haemorrhage. Patients 2 and 3 died in refractory cardiogenic shock despite IABP support. In patient 2, transcatheter VSD closure in the acute phase was attempted but frequent episodes of ventricular tachycardia followed by ventricular fibrillation occurred when the exchange wire was introduced across the VSD into the right ventricle and the procedure was terminated. In patient 3, who had a 22-mm defect measured from the left ventriculogram, transcatheter VSD closure had to be cancelled because a sufficiently large device was unavailable in China at that time. This patient underwent an initially successful surgical repair but died of refractory heart failure two weeks after cardiac surgery. Patient 4 died due to refractory ventricular arrhythmia after successful transcatheter VSD closure and PCI therapy. Patient 5 died of acute renal dysfunction induced by more than 300 ml contrast medium used in separate successful PCI and failed to undergo the transcatheter VSD closure for the second ventricular defect.
OUTCOMES OF TRANSCATHETER VSD CLOSURE
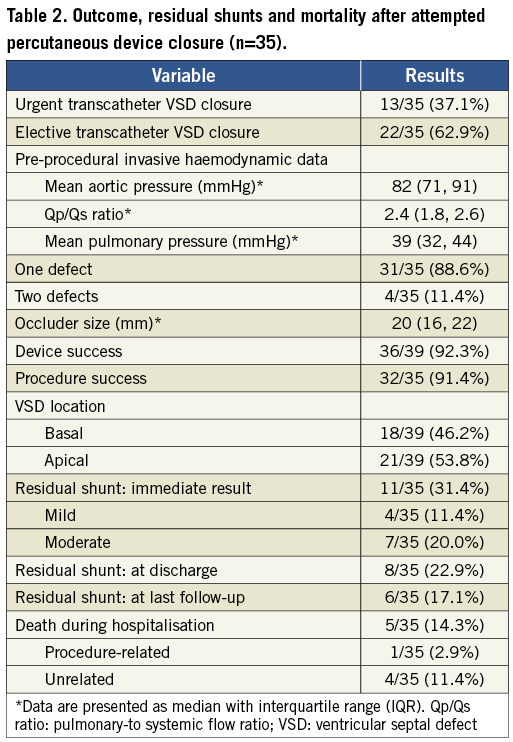
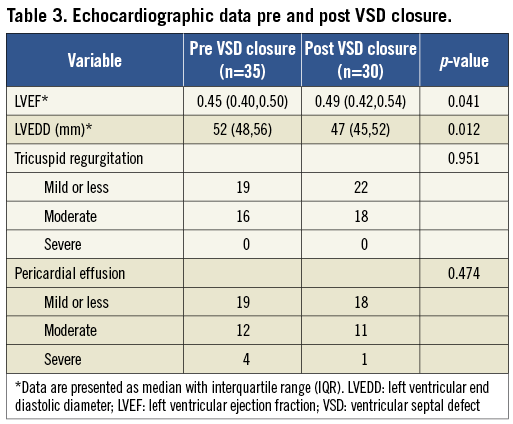
The median length of time from VSD diagnosis to transcatheter VSD closure was 18 days (IQR: 4-32). Thirteen patients who had cardiogenic shock with severe hypotension supported by IABP underwent transcatheter VSD closure within two weeks (median: 5 days [IQR: 2-7 days]), while the others underwent elective VSD closure at a median of 23 days (IQR: 18-36). The VSDs were located apically in 21 patients and basally in 18 patients. Thirty-two patients underwent successful transcatheter VSD closure including three patients with two defects each which were closed by using two separate PIMVSD devices; as mentioned above, the third patient with two defects had a failed VSD closure in one of his double ventricular defects. The device success rate was 92.3% (36/39) and procedure success was 91.4% (32/35). In the 39 defects of the 35 patients, 34 were tubulous and five were funnel-shaped, as demonstrated by left ventriculography. The median diameter of the 34 tubulous defects was 11 mm (IQR: 7-14). A residual shunt was noted at the time of procedure in 11 patients. Four patients had mild residual shunts (shunt beam <2 mm) and three of these disappeared at the time of discharge, while seven had moderate shunts (shunt beam 2 to 4 mm). No haemolytic anaemia was found in these patients. Outcomes of transcatheter VSD closure are summarised in Table 2 and echocardiographic data of pre-VSD and post-VSD closure are listed in Table 3.
OUTCOMES OF PERCUTANEOUS CORONARY INTERVENTION
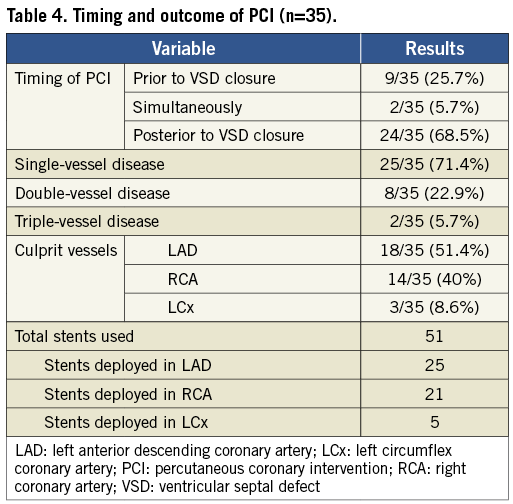
The median duration from AMI symptom onset to PCI was 24 days (IQR: 7-36). The PCI procedures were performed using the standard femoral (n=18) or radial (n=17) approach. Among 35 culprit vessels, 26 were patent at the index PCI procedures, and ischaemia was proved in the territory of nine other occluded vessels by either single-photon emission computed tomography or echocardiography. The lesions were revascularised by implantation of 51 coronary stents with a mean of 1.46 stents per patient. The stent size used was 3.4±0.5 mm in diameter and 27.2±13.1 mm in length. The immediate PCI success rate was 100% without major procedural complications. The outcomes and the timing of PCI are summarised in Table 4. Complete revascularisation (all stenosis >50% in vessels >2.5 mm in diameter was reduced to <50% diameter stenosis) was achieved in all patients.
LONG-TERM FOLLOW-UP DATA IN PATIENTS WHO UNDERWENT TRANSCATHETER THERAPY
The LVEF measured by echocardiography increased from 0.45 (IQR: 0.40-0.50, n=35) at admission to 0.49 (IQR: 0.42-0.54, n=30) at discharge (p=0.041), while the LVEDD decreased from 57 mm (IQR: 52-62, n=35) to 52 mm (IQR: 48-56, n=30) (p=0.012). The median follow-up time from discharge was 53 months (IQR: 9-70; ranging from 2 to 80 months). At the most recent outpatient follow-up, there were two cardiac deaths, which occurred at 38 and 41 months after discharge, respectively. The chest radiograph in all survivors showed the VSD devices and stents placed in situ without device migration. Transthoracic echocardiography demonstrated the VSD to be completely closed in two of the eight patients who had a moderate residual shunt at the time of discharge (organised thrombosis around the closure device was the probable cause), and only a mild residual shunt left in the other six patients. The LVEF of the 30 patients who were discharged alive further increased from 0.49 (IQR: 0.42-0.54, n=30) at discharge to 0.53 (IQR: 0.50-0.56, n=28) (p=0.036) and the LVEDD further decreased from 52 mm (IQR: 48-56, n=30) to 47 mm (IQR: 45-52, n=28) (p=0.030) at the latest available follow-up. The patients who survived the perioperative period have had a three-year survival rate of 100% (n=30) and five-year survival rate of 93.3% (28/30).
Discussion
Transcatheter closure of congenital VSD has become a feasible and safe clinical therapy in recent years, and advances in interventional equipment and experience have made transcatheter closure of VSD complicating AMI an alternative therapy to surgical repair5-7,21. In this series, a total of 35 patients from five Chinese heart centres underwent attempted transcatheter VSD closure and PCI therapy for the purpose of treating VSD complicating AMI. Twenty-eight patients were alive and their status was good at the median duration of 53 months follow-up. The overall mortality in our study was lower than the previously reported mortality for concomitant surgical repair of post-infarction VSDs and coronary artery bypass grafting23,24.
As it is believed that shortly after AMI the ruptured myocardium is too fragile to be safely repaired, a waiting period of three to six weeks is necessary to allow the infarct muscle to develop a relatively firm scar so that the defect can be repaired or closed25. Furthermore, after the oedema has faded, the defect measurement will be more accurate. Nevertheless, the higher survival rate after delayed surgery reflects a survivorship bias in that sicker patients have already died prior to that time. However, the in-hospital mortality was significantly higher in patients who presented with cardiogenic shock with a concomitant VSD than that caused by other AMI complications. Many patients with cardiogenic shock die while awaiting surgical repair or transcatheter closure of the defects. In view of the poor prognosis of VSD patients and the benefits from VSD closure, transcatheter closure or surgical repair of the VSD in the acute phase is usually the only option in order to stabilise haemodynamics and restore cardiac function for those with unstable haemodynamic status, despite high risk and mortality in both therapeutic operations. Recently Thiele et al21 reported the largest experience to date of primary transcatheter closure of a post-infarction VSD in the acute setting, and the overall 30-day mortality rate in their study remained high (65%), similar to the studies of surgical closure in the acute setting. In our series, among the 22 patients who had relatively stable haemodynamics and who underwent elective VSD closure three weeks after VSD, only two died and the status of the remaining 20 was good at the latest follow-up, while among 13 patients with cardiogenic shock who underwent attempted transcatheter closure of the VSD in the subacute phase, three died shortly after the surgical repair or failed transcatheter closure procedure, respectively. One of the significant discrepancies between their study21 and ours was the selection of patients. In their study, the time from VSD occurrence to percutaneous device closure of “all-comers” was one day21, but it was 18 days in our study. Although current guidelines of the American College of Cardiology/American Heart Association for the treatment of patients with AMI recommend immediate operative intervention in patients with VSD regardless of their clinical status26, in view of the significantly higher risk of urgent VSD closure in comparison with elective closure, the timing of transcatheter VSD closure should depend on the haemodynamic status of the patient. When the patient is haemodynamically stable or has become stable after medication or mechanical support with IABP, closure should be delayed until the oedema has faded and the margins of infarcted muscle have developed a relatively firm scar27. Besides, organised thrombus around VSD would be more mature at the time of a delayed closure procedure and it would be safer for patients to receive closure device implantation.
To date, it is inconclusive as to whether revascularisation of the culprit vessel through emergency PCI could prevent occurrence and expansion of VSD. Based on the fact that reperfusion may rescue the ischaemic myocardium and prevent infarction extension28, we performed early PCI therapy prior to transcatheter VSD closure when there was evidence of severe myocardial ischaemia at the time of admission after AMI, unless the haemodynamic status was unstable. In this study, we did not find any re-rupture of closed VSD reported from surgical therapy8, which may be partly due to the opening of occluded or significant stenotic coronary arteries as well as minor injuries caused by occluders to myocardium. There were no major clinical events in patients who still had residual shunts at discharge. We did not observe any progress of those shunts during follow-up; on the contrary, some of them turned out to be smaller or fully closed. In the patients with residual shunts, the effects of dual antiplatelet therapy on the healing of those shunts remain unknown. This still needs to be assessed in future studies. Some concerns might arise regarding the efficacy of delayed PCI in those patients. However, the presentation of all patients in our study was unstable and some patients had patent culprit vessels with severe stenosis. Therefore, PCI was still potentially beneficial in these patients though the procedure was more likely to be delayed. Further studies are necessary to elucidate whether revascularisation would result in improved clinical outcomes.
Limitations
Several limitations were noted in the present study. First, the study included a relatively small number of patients, which is a reflection of the infrequent observation of post-infarction VSD in the current era of thrombolytic and interventional treatment of AMI. Patients were enrolled only if they agreed to undergo both VSD closure and PCI procedures. To the best of our knowledge, however, the current study represents the largest patient series with the longest follow-up reported so far regarding combined transcatheter VSD closure and PCI therapy to treat VSD complicating AMI. Second, the study lacks randomised comparisons between transcatheter therapy and surgical methods. However, a recent large registry study showed that operative mortality was 54.1% (1,077 of 1,990) if repair was within seven days from MI and 18.4% (158 of 856) if more than seven days from MI29. Based on low mortality in the primary data of the study, it may be concluded that transcatheter therapy is a promising alternative therapy in treating these patients.
Conclusion
Transcatheter VSD closure with concomitant PCI therapy provides a therapeutic option for selected patients with VSD complicating AMI with satisfying long-term efficacy. This procedure can be performed successfully in patients with anatomically suitable VSD and coronary lesions. The timing of VSD closure and PCI therapy should be based on the haemodynamic status of the patient and the severity of the condition of the target coronary vessel.
Conflict of interest statement
The authors have no conflicts of interest to declare.

