Abstract
Ventricular septal rupture (VSR) post acute myocardial infarction is associated with a high mortality rate if not treated. Early surgical repair is recommended regardless of haemodynamic status. We review the role of transcatheter device closure for VSR, which has emerged as an alternative to surgery. The procedure itself has a high technical success rate with a relatively low complication rate; however, it is associated with high in-hospital mortality rates when performed in the early phase. Results post transcatheter closure are best in the subacute phase post MI (in part reflective of a selection bias), or post surgical repair with a patch leak. Transcatheter closure can otherwise be considered in patients who are not surgical candidates, who generally have haemodynamically tolerable defects, and who survive a period of watchful waiting. In general, a hybrid strategy of early surgical closure with transcatheter device closure of a patch leak (if it occurs) is a favoured strategy. Long-term outcomes appear good in patients with or without prior surgery who survive to hospital discharge.
Background
Ventricular septal rupture (VSR) is among the most feared and deadly mechanical complications of acute myocardial infarction (AMI). It has a bimodal occurrence, with peak frequencies in the first 24 hours and at day 3-5 post AMI. The incidence of this lethal complication has become less common with the introduction of reperfusion therapy (reduced from 1-3% to 0.2-0.5% in the current era)1,2. Risk factors for VSR occurrence include advanced age, female gender, and anterior or LAD territory infarction. Conservative therapy invariably leads to death (90-95% mortality within two months of diagnosis without intervention). Surgery has been considered the mainstay of treatment but is often deferred owing to the common associated issues of poor clinical status (especially shock), pulmonary overcirculation, and multiorgan failure.
Current ACC guidelines recommend early surgical repair, regardless of haemodynamic status. Common practice, however, is to defer surgical repair in favour of further medical stabilisation and haemodynamic tailoring; this can at times turn into weeks for patients not quickly accepted. The goal of deferral is to allow myocardial remodelling and tissue healing, particularly at the margins of the defect; however, the consequence is a process which allows patients who declare themselves clinically as survivors to obtain definitive therapy while those who are moribund do not. This process clouds interpretation of surgical series that suggest a benefit for surgical repair over medical therapy or that delayed surgery improves operative mortality3-6. Without knowing the true denominator (i.e., all those presenting with VSR), it is difficult to define accurately the magnitude of benefit associated with surgical repair. It is more correct to consider surgical results as “the mortality among patients offered surgery”. In any case, mortality rates remain high post surgical repair (20-77% in recent series)7, as do recurrent shunting rates, which often relates to patch leaks as sutures pull away from infarcted tissue.
Transcatheter therapy with device occlusion has been explored as an alternative management option in several scenarios: 1) as an acute therapy immediately after VSR is noted (within 3-5 days), 2) as a subacute therapy after allowing tissue remodelling and fibrosis, or 3) as a salvage therapy post patch repair/infarct exclusion where a residual substantial shunt exists. It is important to understand to which of these three groups patients belong when evaluating outcomes.
Initial management post ventricular septal rupture
Initial medical therapy for post-infarct VSR should centre on haemodynamic stabilisation and afterload reduction with vasodilators if possible. Fifty to seventy percent (50-70%) of patients have multivessel coronary disease, thus hypotension with reduced coronary perfusion should be avoided. Intra-aortic balloon counterpulsation can be useful to achieve afterload reduction whilst improving coronary perfusion. A PA catheter is helpful to optimise Qs (systemic flow). While previous focus has been placed on reducing the Qp:Qs ratio, it is in fact optimisation of Qs using pharmacologic means or with haemodynamic support that affords survival to definitive therapy.
Revascularisation of the infarct-related vessel remains controversial in this setting. Angioplasty of an artery supplying a full thickness infarct is generally considered poor practice whilst exposing the patient to unnecessary risk. However, reperfusion may, in theory, improve viability of the defect margins by establishing blood flow to areas of watershed ischaemia, and, interestingly, lack of reperfusion was associated with higher mortality post device closure of VSR in a UK series8. It is worth factoring into the requirement for dual antiplatelet therapy post PCI that can be a deterrent for surgeons and lead to unnecessary delays to operative repair.
Case reports exist describing mechanical circulatory support in this setting, with either Impella® (Abiomed, Danvers, MA, USA), TandemHeart® (CardiacAssist, Inc., Pittsburgh, PA, USA) or extracorporeal membrane oxygenation (ECMO) use as a bridge to transplantation9 or definitive repair, but these are not necessarily practical solutions.
Transcatheter closure of PIVSR
Device closure of post-infarct ventricular septal rupture (PIVSR) has emerged as an alternative to surgical repair. The first report by Landzberg et al10 in 1998 described the use of Clamshell devices (C.R. Bard, Inc., Murray Hill, NJ, USA) or the CardioSEAL® device (NMT Medical, Boston, MA, USA) in 18 patients. Seven patients underwent primary device closure with survival to discharge in three patients, all of whom had presented months after VSR. Better outcomes were seen in the 11 patients who had prior surgical repair with patch leaks (median survival 54 months). More recent series report the use of a dedicated AMPLATZER™ post-MI VSD occluder (St. Jude Medical, St. Paul, MN, USA)7,8 or similarly designed devices7.
The procedure itself is demanding but less complicated than non-infarct VSD closure. Issues that plague early surgical repair, in particular fragile myocardial tissue at the defect margins, are also relevant to percutaneous closure. A device may be successfully implanted, only to place tension on poor quality adjacent tissue that had yet to liquefy and cause VSR expansion. Abnormal geometry is common and can complicate device closure (one autopsy series identified only 53% of VSR being “through and through” while the remainder were complex and serpiginous11, such that the LV entry and RV exit are distant from each other). Furthermore, it is not uncommon for these infarcts to be in locations that abut the free wall or apex that may prevent devices from sitting in a manner whereby they are most effective.
EVIDENCE FOR DEVICE CLOSURE
Important aspects to consider when reviewing device series for PIVSR closure are the timing of the closure (acute versus subacute >2-3 weeks) and whether it is performed as a primary VSR closure versus post surgical repair (for patch leaks). Available evidence for transcatheter closure of PIVSR is limited to scattered case reports and a few case series. The largest series are listed in Table 17,8,12-15.
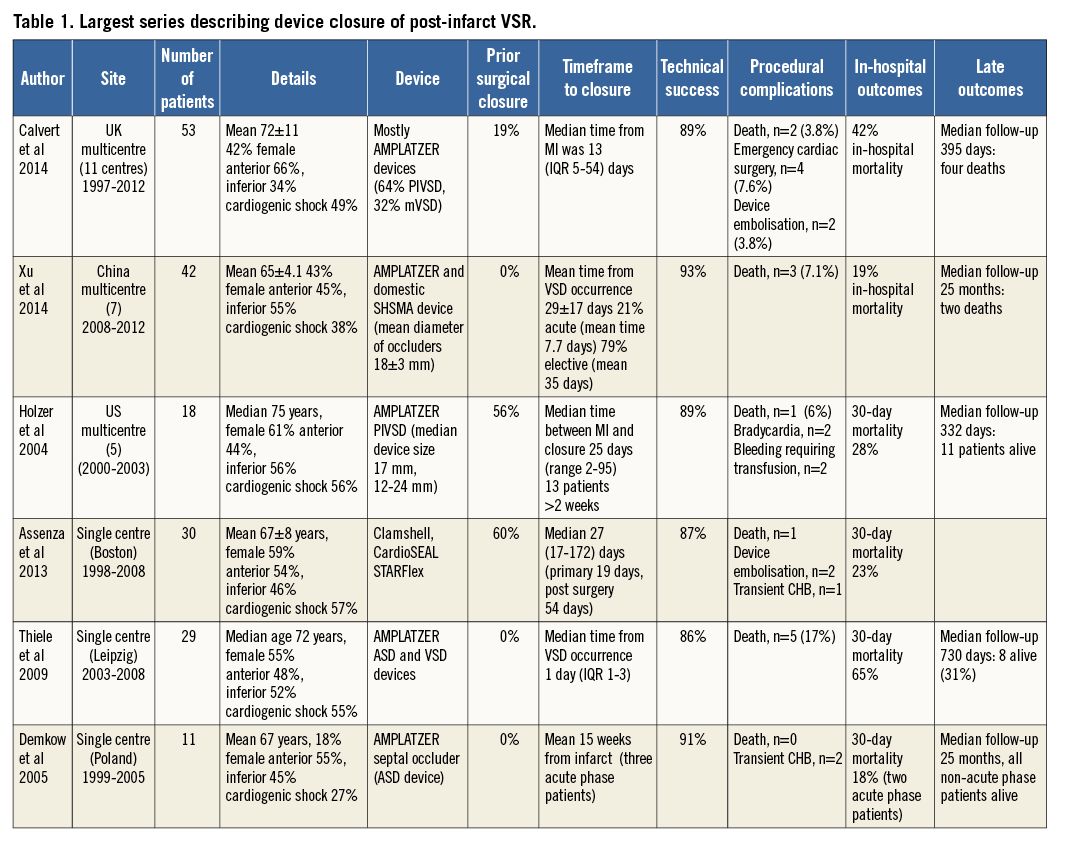
TECHNICALLY FEASIBLE WITH LOW COMPLICATION RATE
Technical success is high in the above case series, ranging from 86-93%7,8,12,14. It would be fair to characterise many of these “successes”, at least in the acute setting, as bittersweet: although a device may have been securely implanted, shunting is often abundant and frequently escapes characterisation. Similarly, views by TEE are often challenging, off axis and are difficult to analyse quantitatively. Success appears to be higher in delayed compared to acute procedures, as witnessed in the Chinese series (97 versus 78%, p<0.01)7. Multiple devices are occasionally required to achieve occlusion of one or multiple defects7,8.
Procedural complications are relatively infrequent. Intraprocedural death occurred in 7% (3/42) of patients in the Chinese series: two deaths were due to LV rupture, thought related to device manipulation, and one to refractory VF during device delivery7. Intraprocedural death occurred in 3.8% (n=2) in the UK series, and emergency cardiac surgery was required in 7.5% (n=4). Five patients in the UK series had repeat procedures for a failure but the outcomes of those procedures were not used in their analysis8. Device embolisation appears relatively infrequent (3.8% in the UK series8 and 5% in the Boston series13).
MORTALITY POST DEVICE INSERTION
Despite a relatively low procedural complication rate, there remains a very high mortality rate post device insertion in the acute phase, as exemplified in the series by Thiele et al14 (comprising only primary closures), where 30-day mortality was 65% in 29 cases where closure was undertaken early post diagnosis. In the Chinese series7, in-hospital mortality was clearly higher in the acute versus elective patients (67% versus 6%). By comparison, the 30-day mortality rate in the series by Holzer et al12 was only 28%, which probably reflects selection bias in the setting of delayed closure given that the majority of closures occurred two weeks post infarction (median 25 days).
In-hospital mortality in this setting is mostly due to multiorgan failure in the setting of cardiogenic shock rather than a direct procedural complication. The MELD-XI score has been used to quantify the degree of organ dysfunction and is a strong predictor of death within 30 days of PIVSR closure13, highlighting the fact that multiorgan failure confers a very high mortality rate irrespective of closure attempt.
Data from the UK series8 clearly suggest that, if patients survive to discharge, they tend to do well; of those that survived in the UK series (66%, n=31), only an additional four patients died during a median follow-up of 395 days. Survival was more likely if prior surgical closure had been performed and if there was an immediate reduction in shunt post closure. Similarly, the Chinese series7 reported a good long-term outlook if survival to discharge occurred: only two patients died and no serious events occurred otherwise during median follow-up of 25 months (range 0-58 months).
PRIMARY REPAIR VERSUS PATCH LEAK
Transcatheter closure of patch leaks post surgical repair is associated with excellent technical outcomes13 and relatively low mortality rates at 30 days8,13 compared to primary VSR device closure. This adds weight to the adoption of a hybrid strategy with early surgery (accepting a higher risk of patch leak with less rigid margins) and subsequent transcatheter closure if required for residual or recurrent defects.
PRE-PROCEDURE PLANNING
Pre-procedure evaluation should include a detailed transthoracic echocardiogram to exclude pericardial effusion, assess LV and RV function, assess the mitral valve and define RV systolic pressure. Multislice cardiac CT can be performed rapidly in unwell patients and is useful to delineate defect anatomy and size, which can define feasibility for closure. The optimal way to display the volume data provided by CT scanning remains to be clarified. The traditional method of displaying three-dimensional data on 2D screens is most familiar to radiologists and some cardiologists; however, the use of rapid 3D prototyping, holographic imaging, or use of a workstation to display and manipulate 3D data sets (e.g., EchoPixel; EchoPixel, Inc., Mountain View, CA, USA) (Figure 1) may provide advantages that are yet to be discovered. Holding a heart model with the defect in question which is built to scale within 12 hours of a scan offers limitless possibilities for testing, device simulation, as well as device development but remains inaccessible to many at the present time.
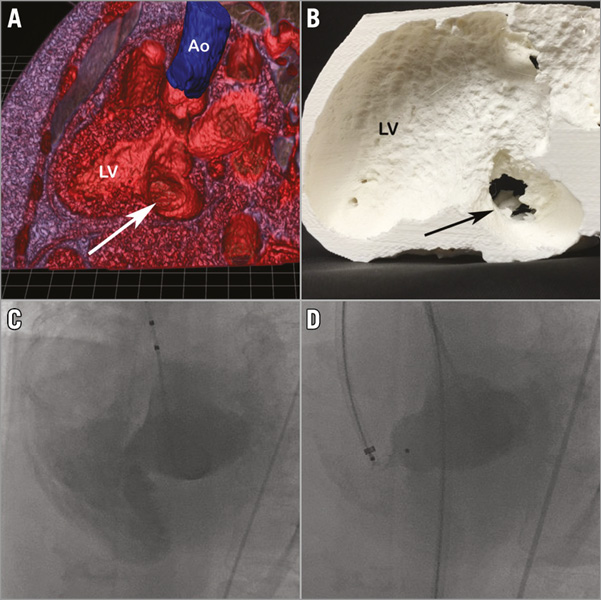
Figure 1. Case example of device closure of PIVSR. A 63-year-old female with a history of multi-infarct dementia presented with an inferior AMI and was treated with primary PCI. An echo the next day revealed a wall motion abnormality with relatively preserved LV function and she was repatriated to her home institution. A VSR was found within several days of her presentation with her course complicated by delirium and a significant intestinal bleed. Creatinine peaked at 156 mmol/L. She was not a candidate for surgery. Device closure undertaken eight weeks post MI. The defect was characterised with TTE and cardiac CT. An EchoPixel 3D reconstruction image (oblique long-axis view) (A; arrow indicates VSR, which measured 14 mm). Model created with 3D printing (B; arrow indicates VSR as visualised from LV with surrounding aneurysm). The procedure was performed under GA with TEE guidance. The defect was crossed from the LV and an AV loop was created. Ventriculography at the time of closure (C). An 18 mm AMPLATZER PIVSD device (30% oversize) was deployed from the venous side successfully (D) with an excellent technical and procedural outcome.
Who should be offered device therapy?
Surgery is preferable to device therapy as primary therapy for PIVSR, in particular for large defects in unstable patients; however, many patients are poor surgical candidates owing to age, comorbidities, multiorgan failure, haemodynamic instability, futility or patient preference. It remains a challenge to identify those patients who are appropriate for surgical repair and will survive a period of observation and scar maturation.
Whilst the majority of patients in the literature are treated subacutely, we believe most patients should have early (within 24-48 hours) surgical intervention if possible according to current guidelines, especially given that rapid and sudden deterioration is common and haemodynamic status pre procedure has a major impact upon surgical outcomes. Device therapy appears well suited for closure of post-surgical VSR patch leaks. Therefore, a provisional hybrid approach seems reasonable with early surgical repair and device closure of a patch leak if it occurs.
Use of device therapy to stabilise haemodynamics as a bridge to surgical repair in very unwell patients with multiorgan failure has been described, but is rarely effective. It remains controversial whether to offer device therapy as salvage to a group of patients who are thought inoperable. Doing so will consistently result in poor outcomes for device therapy, but does that mean we should not offer it? Transcatheter closure can be considered for well-informed patients who are unsuitable for surgery or who refuse surgery and who have appropriate anatomy. It is important to realise, however, that a surgical turn-down does not imply suitability for device therapy and it should be emphasised that most patients treated acutely with primary device therapy will die (usually as an inpatient from multiorgan failure rather than from device-related complications); however, those who do survive tend to do well in the medium to long term. We would consider the presence of a pericardial effusion an ominous sign of impending rupture and to be a serious relative contraindication to device therapy.
Although a variety of devices may be used to close VSR, most experience has been with the AMPLATZER PIVSD device, which has a maximum size of 24 mm. This device is imperfect, requiring a relatively large sheath to deliver, being somewhat stiff and bulky, and often not conforming to the irregular and jagged edges of a VSR. Beyond the jagged edges of these defects lies the challenge of abutting either the apex, mitral valve, or free wall, situations where rigid devices often fail to sit in the orientation for which they were designed and thus leak. As VSR is a problem in decline, the ground may not be terribly fertile for industry to create novel avenues of therapy.
It is important to develop an institutional plan if transcatheter closure of PIVSRs is to be performed. Device closure should be undertaken by interventionalists with structural interventional experience, in consultation with cardiac surgeons, and, ideally, by a dedicated team with a plan a priori of who will be involved. This enhances institutional experience, as these infrequent procedures on sick patients mandate a coordinated team effort for success.
Conclusions
Transcatheter closure of PIVSRs can be performed with high technical success and relatively low procedural complication rates; however, in the acute setting it is associated with very high in-hospital mortality rates. Device closure appears well suited to treatment of patch leaks post surgical repair in particular, thus a hybrid strategy of early surgical repair with transcatheter closure of patch leaks as needed is preferable for primary PIVSRs regardless of haemodynamic status. Transcatheter closure can also be considered in patients who are not surgical candidates, generally have haemodynamically tolerable defects, and survive a period of watchful waiting. Long-term outcomes appear good in patients with or without prior surgery who are treated subacutely and survive to hospital discharge.
Funding
E. Horlick is supported by the Peter Munk Chair in Structural Heart Disease Intervention.
Conflict of interest statement
E. Horlick is a consultant for St. Jude Medical. W. Wilson has no conflicts of interest to declare.

