Abstract
Aims: To test the feasibility of a thoracoscopically assisted, off-pump, transcatheter ventricular reconstruction (TCVR) approach in an ovine model of left ventricular (LV) anteroapical aneurysm.
Methods and results: Myocardial infarction (MI) was induced by coil occlusion of the middle left anterior descending artery and diagonals. Two months after MI creation, TCVR was performed via a minimal thoracotomy in eight sheep. Under endoscopic and fluoroscopic guidance, trans-interventricular septal puncture was performed from the LV epicardial scar. A guidewire was externalised via a snare placed in the right ventricle from the external jugular vein. An internal anchor was inserted over the wire and positioned on the right ventricular septum and an external anchor was deployed on the LV anterior epicardium. Serial pairs of anchors were placed and plicated together to exclude the scar completely. Immediately after TCVR, echocardiography showed LV end-systolic volume decreased from pre-procedure 58.8±16.6 ml to 25.1±7.6 ml (p<0.01) and the ejection fraction increased from 32.0±7.3% to 52.0±7.5% (p<0.01). LV twist significantly improved (3.83±2.21 vs. pre-procedure –0.41±0.94, p=0.01) and the global peak-systolic longitudinal strain increased from –5.64% to –10.77% (p<0.05).
Conclusions: TCVR using minimally invasive access techniques on the off-pump beating heart is feasible and resulted in significant improvement in LV performance.
Introduction
Surgical ventricular reconstruction (SVR) has been proposed as an alternative therapy for the treatment of advanced heart failure patients following large anteroseptal myocardial infarction (MI). Clinical studies have demonstrated that SVR results in restoration of left ventricular (LV) chamber size, with improvement in geometric shape and reduction in myocardial wall stress1-5. Despite the favourable effects of SVR, its widespread use in an advanced heart failure population has been limited by the invasiveness of the procedure: SVR requires cardiopulmonary bypass and a left ventriculotomy. A novel and less invasive, off-pump beating heart, open surgical technique has recently been reported6-8. The procedure is performed via a left thoracotomy or sternotomy with deployment of serial pairs of myocardial anchors (Revivent™ Myocardial Anchoring System; BioVentrix, San Ramon, CA, USA) that effectively exclude the non-contractile portion of the LV. Early studies investigating the Revivent™ Myocardial Anchoring System have reported significant left ventricular volume reduction with improvement in cardiac performance. Utilising the basic engineering principles of this plication anchoring system, the approach for deployment of the anchors has been further refined. A minimally invasive transcatheter ventricular reconstruction (TCVR) system has been developed to achieve similar technical results without the need for a big incision on the chest. The TCVR approach is performed via a minimally invasive thoracotomy under endoscopic and fluoroscopic guidance in a hybrid catheterisation laboratory. In this study, we tested the feasibility and efficacy of TCVR on an ovine ischaemic heart failure model with an anteroapical aneurysm.
Methods
STUDY DESIGN
The study was conducted in accordance with the Guide for Care and Use of Laboratory Animals and was approved by the Institutional Animal Care and Use Committee (IACUC) of the Jack H. Skirball Center for Cardiovascular Research of the Cardiovascular Research Foundation. All animals received humane care in compliance with current guidelines. LV anterior MI was induced in eleven Dorsett hybrid sheep (43.3±5.7 kg) by percutaneous coil embolisation of the middle left anterior descending coronary artery (LAD) and the corresponding diagonals. Eight weeks following MI creation, TCVR was performed through a minimal (4 cm) left thoracotomy and the devices were implanted under endoscopic and fluoroscopic guidance. LV performance was evaluated by echocardiography prior to (baseline) and immediately post device implantation.
DEVICE DESCRIPTION
The transcatheter ventricular reconstruction system consists of several implantable and delivery system components (Figure 1). Similar to the surgical system, the percutaneous system contains a series of titanium anchor pairs covered in polyester. The anchor pairs are connected to each other by a tether made of poly-ether-ether-ketone (PEEK); the separation distance is continuously adjustable and is determined by the location of the sliding (external) anchor relative to the fixed (internal) anchor (Figure 1A). The delivery system comprises an introducer sheath, a snare kit, nitinol transventricular puncture needles (Figure 1B), a needle alignment device, trocars and a tether stabiliser. The top flex arm of the needle alignment device (Figure 1C) is used as an adjustable alignment tool for accurate positioning of the puncture needle between the ribs from outside the chest wall, and the distal suction cup is attached to the epicardial anterolateral LV wall via vacuum and allows accurate as well as secure positioning of the needle and control of the needle-epicardial interface.
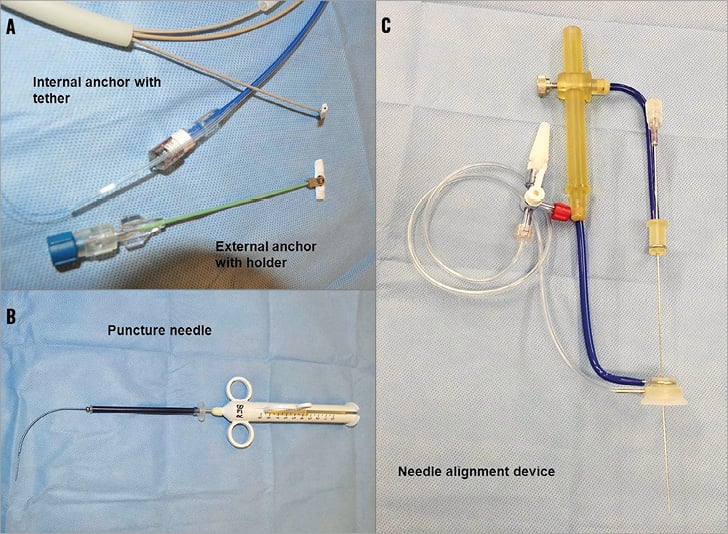
Figure 1. The Revivent™ Myocardial Anchoring System and the transcatheter delivery system. A) Internal anchor with tether and external anchor with holder. B) Puncture needle. C) Needle alignment device.
MYOCARDIAL INFARCTION MODEL DEVELOPMENT
LV anteroapical MI was induced by percutaneous coil embolisation of the LAD6,7. All animals received intramuscular Telazol (4 mg/kg) injections for induction, and were then intubated and mechanically ventilated using 1.5-2.5% isofluorane. Heparin (100 U/kg) was injected to maintain an activated clotting time (ACT) of >250 seconds. Under fluoroscopic guidance, the coronary coil (2.0 to 4.0 mm; Cook Medical, Bloomington, IN, USA) was delivered to the middle portion of the LAD, at a point determined to be forty to fifty percent along the distance of the LAD in an apical to basal line. Additional coils were placed in the diagonals, distribution as necessary. Coronary angiography was performed to confirm complete and persistent coronary artery total occlusion. All catheters and sheaths were removed two hours after LAD occlusion and animals recovered from anaesthesia.
TRANSCATHETER VENTRICULAR RECONSTRUCTION TECHNIQUE
The key procedural steps are illustrated in Figure 2. Eight weeks following MI creation, anaesthesia was induced as described above and the TCVR procedure was performed in eight sheep. A 10 mm thoracoscope (Stryker Endoscopy, San Jose, CA, USA) was placed via a small incision in the eighth intercostal space. The heart was exposed through a minimally invasive left thoracotomy (~4 cm), the pericardium was opened and the LV anteroapical scarred portions were identified. Intravenous heparin (100 U/kg) was administered to maintain an ACT of >250 seconds during the procedure. A needle alignment device was passed through the minithoracotomy incision and the suction component was positioned within the infarct zone on the LV anterolateral epicardium. A trocar locator device was inserted between the ribs to establish proper alignment of a trocar with the needle alignment device (Figure 3A). An 8 mm trocar was inserted and affixed into the suction component on the cardiac surface. Under fluoroscopic guidance, an Agilis™ NxT steerable catheter (St. Jude Medical, St. Paul, MN, USA) with snare (Amplatz GooseNeck Snare Kit; ev3 Inc., Plymouth, MN, USA) was inserted into the right ventricle (RV) (Figure 3B). The appropriate transventricular curved needle was then selected and passed through the trocar and engaged into the centre port of the needle alignment device (Figure 3C). Under fluoroscopic guidance, the puncture needle was advanced across the interventricular septum into the right ventricle and the snare captured the needle tip (Figure 3D). Additionally, the needle was connected to a pressure-transducer (DTX Plus™ DT-XX; BD, Franklin Lakes, NJ, USA) and the respective pressure waveforms of the LV, septum, and RV were recorded to ensure proper advancement. A flexible 0.014’’ guidewire (Roadrunner®; Cook Medical) was advanced via the needle into the RV chamber and pulled out through the right external jugular vein (Figure 3D). Then the internal anchor, affixed on a tether with a monorail system, was inserted over the wire and the leading edge of the tether was advanced across the interventricular septum and out through the LV anterolateral epicardial surface (Figure 3E). Gentle traction on the tether was utilised to pivot the anchor against the right side of the interventricular septum (Figure 3F). Subsequently, the needle alignment device was removed (Figure 3G) and an external locking anchor was deployed over the remaining tether to the LV anterior epicardium (Figure 3G, Figure 3H). This process was repeated with additional pairs of anchors (two to three pairs), aligned in the long axis, to achieve the desired line of apposition between the lateral LV wall and the septum (Figure 3I, Figure 3J). The external anchors were serially advanced towards the internal anchors with a measured compressive force of 2-4 Newtons. The delivered force served to appose the walls and effectively plicate the aneurysm (Figure 3K, Figure 3L). The number of anchor pairs utilised was based on preoperative LV volume and infarct area measurements by echocardiography and visual assessment. The desired goal was either complete scar exclusion or reduction of end-systolic volume by thirty percent. Following device deployment, the endoscope was removed and all animals were euthanised. The hearts were explanted and the implant sites was grossly examined.
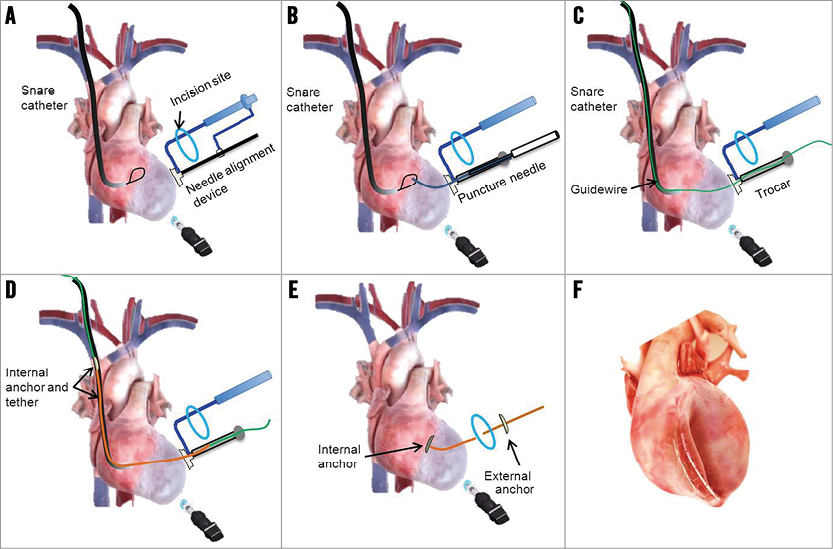
Figure 2. The transcatheter ventricular reconstruction procedural steps. A) The steerable catheter with snare insertion into the RV via the right external jugular vein access and placement of the needle alignment device on the LV epicardium via minithoracotomy. B) Engagement of transventricular needle and entrapment by snare. C) Wire advancement and capture by snare. D) Passage of inner anchor assembly and retrieval through LV wall. E) External anchor placement over the tether. F) Apposition of LV wall with serial pairs of anchors.
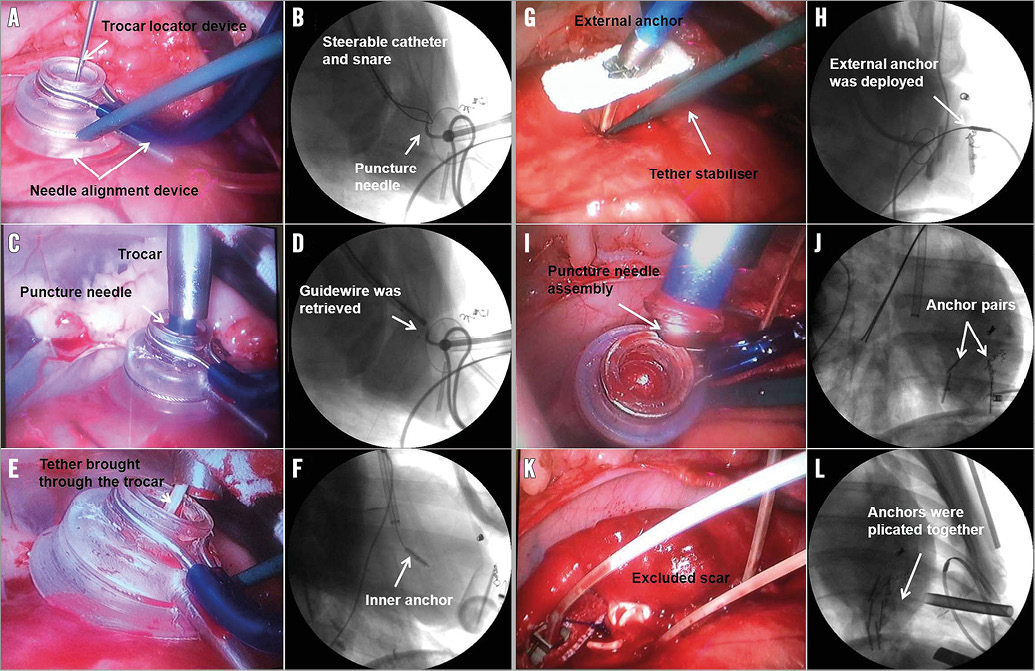
Figure 3. Angiographic and endoscopic images of key operative steps. A) The needle alignment device with tether stabiliser was placed on the epicardium through minithoracotomy. B) A steerable catheter with snare was inserted into the RV via the right external jugular vein. C) Trocar placement and engagement of transventricular needle puncture into the LV chamber. D) A 0.014’’ guidewire was advanced via the needle and captured by snare. E) Internal anchor assembly passage and tether retrieval through LV wall. F) The internal anchor was pulled back to attach the RV septal wall and the needle alignment device was removed; G) & H) The external anchor was placed over the tether. I) & J) Additional pairs of anchors were placed. K) & L) The serial pairs of anchors were plicated together to exclude the scar completely.
ECHOCARDIOGRAPHIC EVALUATION
Echocardiograms were performed prior to (baseline) and immediately after device implantation. Two-dimensional (2D) echocardiographic images were acquired in the right lateral decubitus position using a 5-MHz probe (iE33; Philips Medical Systems, Bothell, WA, USA) from standard parasternal long- and short-axis planes. LV end-diastolic diameter (EDD) and end-systolic diameter (ESD) were determined from short-axis planes; end-diastolic volume (EDV) and end-systolic volume (ESV) were calculated using Simpson’s rule, and LV ejection fraction (EF) was calculated using a standard formula (EF=[(EDV-ESV)/EDV] ×100).
Greyscale images for offline speckle tracking echocardiography (STE) analysis were acquired at a high-frame rate (65-90 frames/s) from standard long-axis and apical four-chamber views. Short-axis images were acquired at three different levels (base, mid, and apex) from standard parasternal views. At least two consecutive cardiac cycles were recorded for offline analysis (QLAB 9.0 software; Philips Medical Systems). When a cardiac cycle with a good quality image was selected, a region of interest for speckle tracking was defined at end diastole using a semi-automated border detection method. The locations of the tracking points extending from endocardial to epicardial borders were adjusted and then the segmental myocardial strain curves were automatically generated by the system. The basal and apical rotations were analysed as previously described6, and the LV peak systolic twist was calculated as the net difference in LV rotation at isochronal time points between the apical and basal short-axis planes. For each animal, the global peak negative systolic circumferential strain was derived from the mean value of all short-axis segments. Peak systolic longitudinal strain in all LV segments (basal septum, mid septum, apical septum, apex, basal lateral, mid lateral and apical lateral) was averaged to obtain a global value (global longitudinal strain - GLS)9.
STATISTICS
Statistical analyses were performed using SAS statistical software version 9.2 (SAS Institute Inc., Cary, NC, USA). The differences within groups at distinct time points were assessed by paired t-test. Mean±standard deviation is presented for continuously distributed variables. A p-value <0.05 was considered statistically significant.
Results
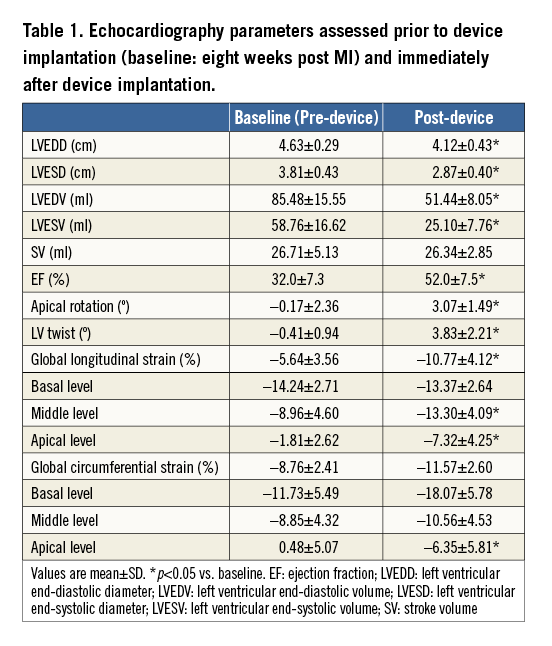
A total of 11 animals underwent MI induction: two (18%) did not survive the perioperative MI induction procedure and nine survived to enrolment in the TCVR procedure eight weeks post MI creation. Preoperative transthoracic echocardiography confirmed the absence of left ventricular thrombus in all animals. Large anterior infarction and apical dyskinesis were observed in all animals, and none had mitral or tricuspid regurgitation. All animals developed heart failure characterised by reduction in ejection fraction (EF) and dilated LV chamber (Table 1).
TRANSCATHETER VENTRICULAR RECONSTRUCTION PROCEDURE
At the time of the TCVR procedure, one animal died due to ventricular arrhythmia when the first transventricular puncture was performed. TCVR was successfully completed in eight cases without adverse haemodynamic consequences. Postoperative echocardiography confirmed that there was no communication between the restored ventricular chamber and the excluded ventricular space and that there was no ventricular septal defect. Echocardiographic data are shown in Table 1. After device implantation and compared to baseline (pre-device), TCVR significantly reduced LV end-diastolic volume (by 39%, p<0.001) and LV end-systolic volume (by 57%, p<0.001). Ejection fraction increased by 20% (52.0±87.5% vs. baseline 32.0±7.3%, p<0.001). Stroke volume was preserved in all animals (p=0.84). No mitral regurgitation was found in any of the animals following device implantation. Mild tricuspid regurgitation was detected in one animal.
All animals had adequate image quality and excellent tracking score for long- and short-axis strain analysis. Eight weeks following MI creation, LV peak-systolic longitudinal and circumferential strains in the apical level were significantly reduced, and dyskinetic contractility was shown in the apex and anteroseptal wall (Figure 4). Immediately after device implantation and ventricle reconstruction, the apical rotation dramatically improved (3.07±1.49° vs. baseline –0.17±2.36°; p=0.03), and in addition the LV twist (3.83±2.21° vs. baseline –0.41±0.94°; p=0.01) recovered significantly. The adverse longitudinal strains in the apex (–4.96±6.03% vs. baseline 0.46±5.13%, p=0.02) and the posterolateral wall (–9.82±8.46% vs. baseline 1.85±7.92%, p=0.02) were reversed (Figure 4A). The circumferential strain in the inferior lateral (–15.45±4.43% vs. baseline –8.77±4.07%, p=0.02) and the anteroseptal wall (–5.23±5.50% vs. baseline –0.71±4.42%, p=0.04) were significantly improved (Figure 4B).
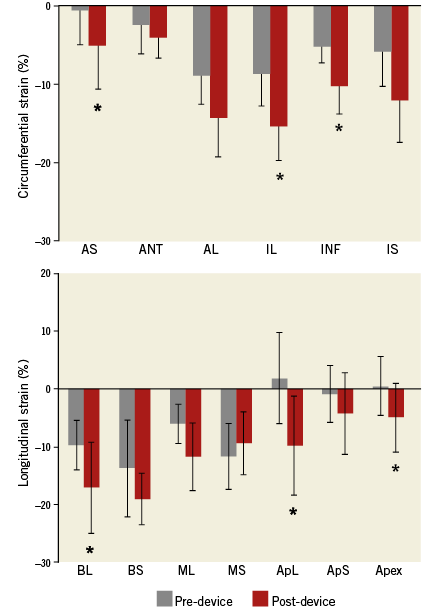
Figure 4. Regional circumferential and longitudinal strain analysis. A) LV peak-systolic circumferential. B) Longitudinal strain changes before and after device implantation. Values are mean±SD. *p<0.05 vs. baseline (pre-device). AL: anterior lateral; ANT: anterior; ApL: apical lateral; ApS: apical septum; AS: anterior septum; BL: basal lateral; BS: basal septum; IL: inferior lateral; INF: inferior; IS: inferior septum; ML: mid lateral; MS: mid septum
GROSS PATHOLOGY EXAMINATION
All subject animals were sacrificed immediately following completion of the post-procedural echocardiogram and were then subjected to necropsy and explantation of the heart. The right ventricle was opened along the inferior septum over its entire length, exposing the configuration of the RV septal anchors. The LV was likewise opened through the mitral valve and along the length of the inferior septum exposing the line of wall apposition. The external anchors were then released along the tethers to allow assessment of the excluded wall. In all cases, a straight line of wall apposition was observed. Since the shape of the scar tended to be irregular, the anchors demonstrated elimination of most but not all of the scar, in keeping with the design for a straight line of wall apposition. Gross assessment of aneurysmal wall exclusion was found consistent with the echo findings, and the remaining ventricular myocardium appeared normal (Figure 5A). Gross examination of the RV demonstrated deployment of the anchors along the anterior septal edges of the scar with no damage to the surrounding tissue in any animals (Figure 5B). In no instance was an anchor placed in normal tissue. In addition, no tricuspid leaflet damage or ventricular septal defects were observed. In an early animal, injury of the tricuspid chordae tendineae was found, which prompted a change in the way the internal anchors were subsequently delivered, given that the anchor caught the cord during deployment.
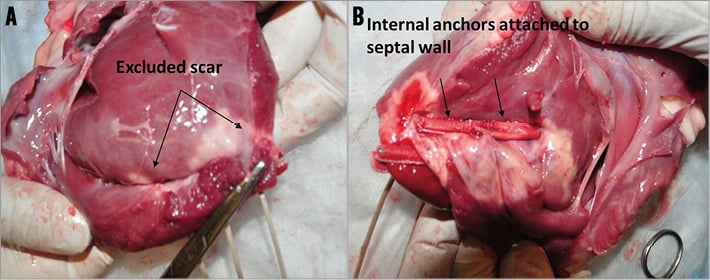
Figure 5. Gross examination demonstrated the implant anchors from the left and right ventricle. A) Implant sites seen from LV showing complete exclusion of the infarcted area and merging with the apposed walls. B) The internal anchors were attached to the septal wall in the RV.
Discussion
Ischaemic cardiomyopathy is the most common cause of cardiomegaly and congestive heart failure. Large-scale clinical trials have demonstrated that SVR procedures are safe and effective in terms of survival benefit and LV functional recovery in patients with ischaemic dilated cardiomyopathy10-12. In addition, SVR has recently been endorsed by the European Task Force on Myocardial Revascularization for consideration as a surgical option combined with coronary artery bypass grafting in selected patients affected by ischaemic heart failure and LV dysfunction13. Our group has recently reported the novel application of an off-pump, epicardial Less Invasive Ventricular Enhancement (LIVE) procedure in an ovine anteroapical aneurysm model without the need for cardiopulmonary bypass6,7. A confirmatory clinical trial8 has also demonstrated the safety and efficacy of this technique in patients with anteroseptal scar and dilated ischaemic cardiomyopathy. In these patients, the LV end-systolic volume index was decreased by 36.2±18.3% (p<0.001) from baseline at six-month follow-up and 39.6±14.8% (p<0.001) at 12 months8. The extent of volume reduction achieved using this novel technology is comparable with multiple observational studies of other ventricular restoration procedures10-12. However, the LIVE procedure requires a sternotomy, and the paired anchors are placed through the 14 Fr introducer delivered directly across the LV anterolateral wall. In the current study, the transcatheter ventricular reconstruction system enabled the internal anchor to be placed via an endovascular platform in a less invasive manner. In TCVR, only the small tether traversed the LV scar versus a 14 Fr sheath in the open chest LIVE procedure, resulting in a marked reduction in the calibre of surgical instrumentation.
Thoracoscope-assisted cardiac surgery has been rapidly developed and successfully performed in the treatment of heart diseases14,15. Compared with conventional surgery, thoracoscope-assisted cardiac surgery involves a significant decrease in surgical trauma, and the potential advantages include less postoperative pain, earlier mobilisation, lower overall morbidity, a shortened hospital stay with reduced costs and reduced operating time16. In our study, the incision located beside the sternum was reduced to 4 cm, and the LV reconstruction procedure inside the chest cavity was performed with small-profile devices under endoscopic and fluoroscopic guidance, further decreasing the degree of surgical manipulation. TCVR was successfully performed using this minimally invasive access in eight animals without cardiopulmonary bypass or left ventriculotomy. Similar to our previous less invasive open chest surgical procedures, significant reductions in LV chamber size and volumes were achieved immediately after device implantation (Table 1).
In the present study, the anteroapical myocardial infarction caused LV dilation and remodelling without MR development in all animals. TCVR restored LV shape, reduced LV volume, and improved pump function with no MR observed immediately after the procedure. Mild tricuspid regurgitation was found in one animal due to excess attempts to insert the snare catheter into the RV probably resulting in damage to the chordae of the tricuspid valve. This was an isolated event and did not occur in any other animals.
In this study, we used clinically validated echocardiographic variables to evaluate myocardial performance. These included LV strain and myocardial twist which are accurate methods for assessing LV function in patients with heart failure17,18. Several studies have shown that LV torsion represents a critically important mechanism for both ejection and filling of the LV19-21. Also, histological studies have demonstrated that myofibre orientation in the thickness of residual normal myocardium is not changed after myocardial infarction22, and the transmural course of fibre orientation angles near infarct zones was similar to that of normal myocardium23. Unlike the surgical left ventricular reconstruction24 and the percutaneous left ventricular partitioning device (Parachute; CardioKinetix, Inc., Menlo Park, CA, USA), the feasibility of which has been demonstrated in clinical trials25, more information is needed to present the thrombus, endocarditis, myocarditis, and dislodgement risks with a long-term implantable device in the LV chamber. Our study showed that apical rotation and LV torsion were significantly improved after TCVR, resulting in significant improvement in LV contractility. Thus, we hypothesise that successful delivery of the TCVR system achieves ventricular reconstruction by circumferentially excluding the non-functional scar from both anterior and septal walls, realigning the anatomically normal fibres of the residual myocardium and restoring more normal physiology. The evolving TCVR procedure has progressed towards an increasingly physiologic restoration of ventricular shape and volume, with increasing attention being paid to the multilayered structure of myocardial fibres. Similarly, it has been reported that reduced longitudinal and circumferential myocardial strain has been strongly associated with higher risk of all-cause mortality among heart failure patients26. Similar to the previous surgical procedure6,7, our present study showed that longitudinal and circumferential strains were significantly improved after TCVR: the greatest regional strain improvements were found at the apical level and inferior lateral wall.
Limitation
The limitations of preclinical animal studies are well known. The animal model does not perfectly mimic human pathophysiology because the interval between infarction and TCVR does not allow adequate time for the adverse remodelling process to affect the remaining myocardium. Also, the scar is very homogeneous, unlike the mosaic distribution most commonly observed in patients. The ovine model is widely used in heart failure studies, but morphological differences in RV configuration as well as the position of the heart in the chest and the structure of the chest wall also present limitations in such a study. In addition, for practical reasons, a small number of animals were tested, and chronic long-term survival data are absent. However, similar acute volume reduction and cardiac performance improvement were found following TCVR as in previous surgical procedures. Future human clinical investigations are necessary to determine the clinical benefit of TCVR with the plication anchoring system.
Conclusion
In summary, thoracoscopically assisted TCVR is a minimally invasive off-pump treatment option that is feasible and safe compared to the conventional surgical ventricular reconstruction approach. Similar to the previously reported surgical approach, TCVR achieved significant improvements in ventricular geometry and overall myocardial performance. Due to the minimally invasive nature of the procedure, it is expected that this technique can be applied to a larger number of critically ill patients and potentially reduce the frequency of complications seen with the surgical approach.
| Impact on daily practice The thoracoscopically assisted transcatheter ventricular reconstruction procedure is an alternative to the conventional surgical ventricular restoration approach, enabling a minimally invasive closed-chest access technique, without the need for sternotomy or ventriculotomy, on the off-pump beating heart. The proprietary transcatheter delivery system enables left ventricular volume reduction via placement of anchors designed to exclude scarred myocardial tissue from the left ventricle while also restoring its more natural conical shape. |
Acknowledgements
The authors would like to acknowledge Adrienne Dardenne, DVM, and Patricia Mount, BS, for their excellent technical assistance. This work was supported in part by BioVentrix (San Ramon, CA, USA).
Conflict of interest statement
L. Annest and K. Van Bladel are employees of BioVentrix. R. Brown and A. Wechsler are consultants of BioVentrix. The other authors have no conflicts of interest to declare.

