Abstract
Aims: Parachute® implantation (PI) is an attractive treatment option for patients with left ventricular apical aneurysms (LVAA). So far, only the retrograde approach has been approved for PI. Unfortunately, severe functional mitral regurgitation (MR) restricts PI. Thus, we were intrigued to combine PI and MitraClip therapy (MCT) as a new transvenous hybrid concept.
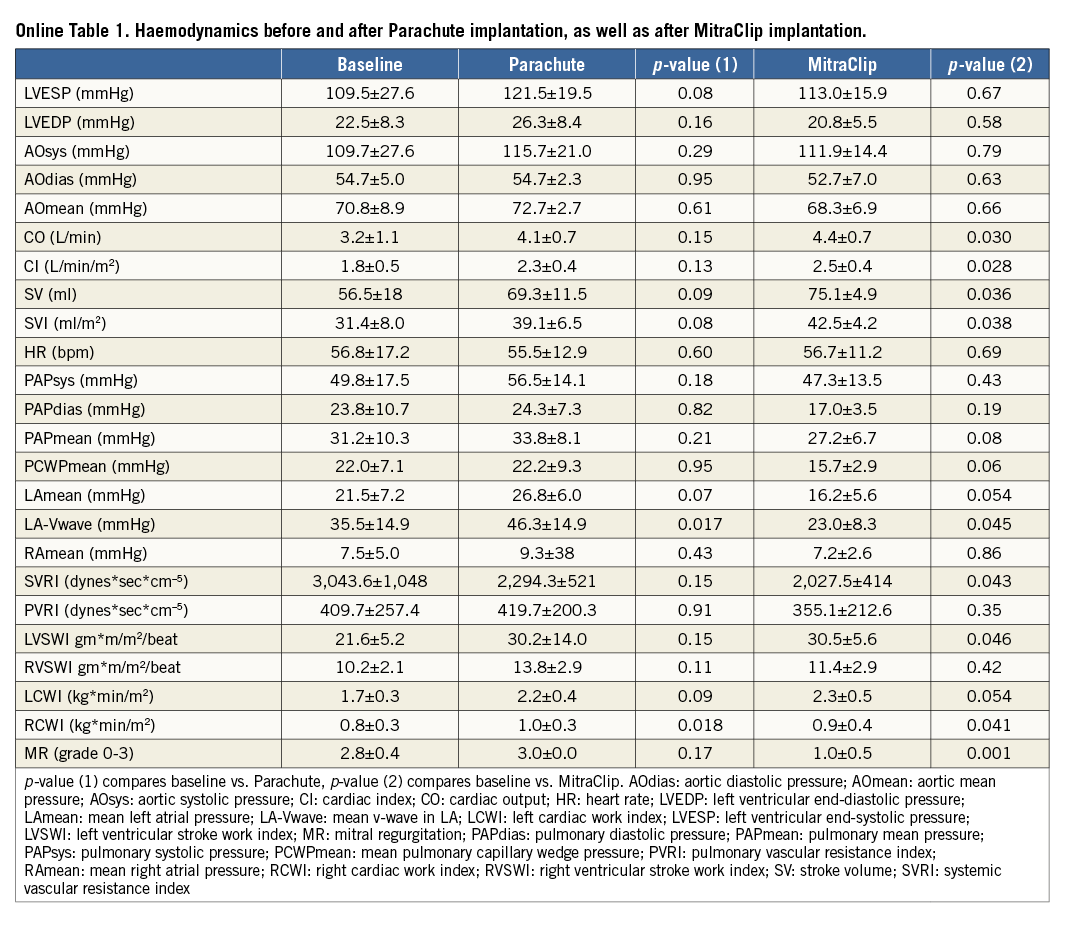
Methods and results: PI was performed via a transseptally placed MitraClip guide in six consecutive patients (age 73.8±5.2; 66% male). Immediately after PI, MR was treated by MCT. Invasive right and left heart haemodynamics were taken before and after PI and MCT, respectively. Procedural success was 100%. PI induced a numerical increase in cardiac output (CO: +36.4; p=0.15) and stroke volume (SV: +30.1%; p=0.09), despite some evidence of MR aggravation. Subsequent MCT successfully reduced MR at least to mild in five patients and to moderate in one patient. SV and CO demonstrated a further increase (SV: +44.3%, p=0.03; CO: +44.5%; p=0.03).
Conclusions: The study documents for the first time the feasibility of transseptal and transmitral PI. Nevertheless, pre-procedural MR seems to counteract the beneficial effects of PI. Hence, the combined transseptal approach of PI and MCT seems to be the appropriate strategy in patients with significant MR and LVAA.
Introduction
Myocardial infarction (MI) is frequently followed by left ventricular remodelling and heart failure, which leads to increased long-term morbidity and mortality1-6. Pharmacological treatment improvements of acute MI have led to larger numbers of survivors7,8, but roughly one quarter of the patients with myocardial infarction seem to develop heart failure9. As a maladaptive consequence, left ventricular (LV) volumes increase, filling pressures rise and stiffening of the ventricular walls will lead to progressive systolic dysfunction2,10-12. In addition, aneurysmatic changes in LV geometry are known to increase myocardial wall stress and oxygen consumption unfavourably, due to an altered temporal distribution of wall stress within the border zone of the LV aneurysm13. Hence, abnormal regional myocardial function of non-ischaemic regions, remote from the aneurysm, is potentially correctible, if the abnormal mechanical burden imposed on the wall is relieved14-16.
For many years, surgical aneurysmectomy was the most applied solution to decrease the size of the ventricular cavity in patients with left ventricular aneurysms17-21. Excision of the infarcted wall in these patients was performed to decrease end-diastolic volumes, reduce myocardial work and wall stress and to receive a haemodynamic benefit. Despite a high rate of technical success, operative mortality was rather high (range 7.7%-17.8%), with a five-year survival rate of 60%22,23.
As opposed to surgical endoventriculorrhaphy and aneurysmectomy with the need of thoracotomy, cardiopulmonary bypass and cardioplegia, the Parachute® ventricular partitioning device (CardioKinetix, Inc., Menlo Park, CA, USA) can be implanted via a percutaneous catheter approach, as a beating heart intervention under conscious sedation. The Parachute device is able to exclude the damaged muscle effectively, leading to an isolation of the non-functional muscle from the functional part24-32.
As a consequence, wall stress in the upper part of the chamber can be reduced and an improvement in compliance and LV filling pressures has been assumed. Animal data have shown an improvement in mechanical efficiency (external work/[external work+potential energy]) by 22%, with a significant shift of pressure-volume loops to the left ventricle27,33. Very recently, similar improvements in haemodynamics and ventricular performance after Parachute implantation have been demonstrated in humans32. Until now, Parachute implantation was performed exclusively via a retrograde transfemoral approach. In addition, significant pre-procedural mitral regurgitation (MR) is considered as a contraindication for Parachute implantation. Due to the relatively high prevalence of significant MR in patients with congestive heart failure and left ventricular apical aneurysms, we were intrigued to use a novel transseptal approach for Parachute implantation in conjunction with MitraClip (Abbott Vascular, Santa Clara, CA, USA) implantation.
Methods
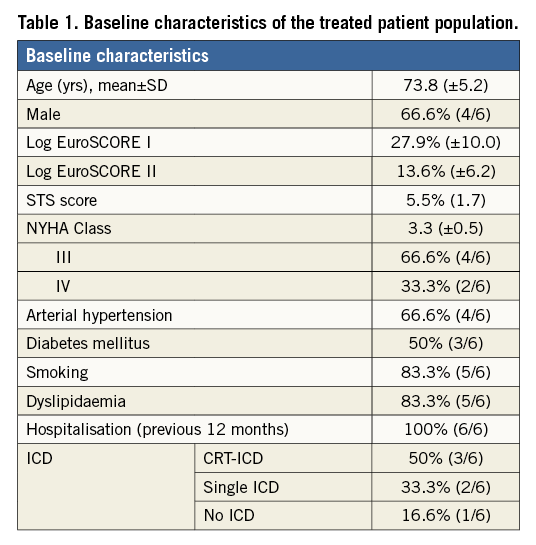
Between September 2012 and March 2015, 49 patients with an LV aneurysm were screened for Parachute implantation. Of these, 32 (65%) were approved on the basis of anatomical considerations (MSCT), and so far 23 of these patients have undergone a planned Parachute implantation at two institutions (same operator). Amongst these, six patients were treated through a transvenous access. All patients were assessed for clinical and echocardiographic baseline data (global left ventricular function and the absence of pathomorphological structures such as pseudo cords, calcification in the landing zone) and all patients underwent a full cardiac cycle MSCT scan. Due to the dynamic nature of MR, each study patient had to have a least one echocardiography demonstrating severe MR in the past with persisting dyspnoea (NYHA >II). All patients were discussed by a multidisciplinary Heart Team, and the study was approved by an institutional review committee. In addition, all subjects selected for a transseptal approach specifically had to give informed consent for this novel access technique.
DEVICE DESCRIPTION AND PROCEDURAL DETAILS
The Parachute device comprises a fluoropolymer membrane stretched over nitinol struts. The device is currently available in four sizes: 65 mm, 75 mm, 85 mm and 95 mm, and two different foot heights (standard and short). It is currently delivered with a guide catheter (available in 14 Fr and 16 Fr) for a transfemoral arterial and retrograde approach. In addition, it contains a specific delivery system with a pre-mounted balloon for device expansion.
After informed consent for the transvenous and transseptal approach had been obtained, all patients were treated under general anaesthesia in a hybrid operating room. Per protocol, we used general anaesthesia (GA) in all patients. General anaesthesia consisted of the volatile anaesthetic sevofluorane with age-adjusted controlled minimal alveolar concentrations (MAC 0.8-1.4 vol%) and the analgesic drug remifentanil at a dose of 0.3-0.5 µg/kg/min, to minimise cardiovascular side effects as much as possible. Moreover, all patients were kept on constant doses of catecholamines (if needed) with the exclusion of inotropic agents. Last but not least, the volume status was kept constant and monitored by central venous pressure. The implantation of the Parachute device was guided by left ventricular angiography as previously described30,34. In contrast to the standard approach32, the combined transvenous, transseptal approach (in conjunction with a MitraClip procedure) was performed through a 24 Fr MitraClip guide. The transseptal puncture was performed in accordance with the IFU of the MitraClip procedure (i.e., puncture in the posterior aspect of the fossa ovale and 4.0 to 4.5 cm above the mitral valve [coaptation zone]). Immediately after transseptal puncture, 100 IU/kg of unfractionated heparin was administered to achieve an activated clotting time of 250-300 seconds. After insertion of the 24 Fr MitraClip guide, a specific flush-line (10 Fr sheath with a three-way stopcock) was installed to prevent air entrapment in the MitraClip guide (Online Figure 1). Following that, a regular pigtail catheter was advanced with a standard 0.035’’ wire in the left ventricular apex. Care was taken to prevent an entanglement in the subvalvular mitral apparatus. Subsequently, the wire was exchanged for a stiff wire (Amplatz Super Stiff™ ST1; Boston Scientific, Marlborough, MA, USA) and the 68° Parachute guide was inserted inside the 24 Fr MitraClip guide (“mother and child” technique) and placed across the stiff wire in the left ventricular apex. After removal of the introducer, the Parachute implantation was performed, similar to the standard approach, under the guidance of fluoroscopy and contrast injections. A perfect implantation angulation could easily be obtained with the steering knob of the MitraClip guide (±knob) under the guidance of 3D TEE imaging (surgical mitral en face view). By clockwise and counter-clockwise rotation of the MitraClip guide, additional steering to the posterior or anterior aspect of the apex could be obtained. Last but not least, pushing the MitraClip guide inside the patient allowed for lateral positioning, and pulling the guide out of the patient allowed for a more medial position (similar to the MitraClip procedure itself). Having verified a perfect implantation position, the device was unscrewed and the Parachute guide was removed from the MitraClip guide. After a left ventricular angiography and haemodynamic assessments, MitraClip implantation was performed as previously described35-37.
INVASIVE HAEMODYNAMIC ASSESSMENTS
Invasive haemodynamic measurements were performed with a Swan-Ganz catheter and a pigtail catheter placed in the left ventricle. Before and after Parachute implantation as well as MitraClip implantation, invasive haemodynamic measurements were performed in all patients to assess acute haemodynamic changes. Measurements of the systolic, diastolic and mean pulmonary artery pressure (PAPsyst, PAPdiast, PAPmean), pulmonary capillary wedge pressure (PCWP), right atrial pressure (RAP), cardiac output (CO), cardiac index (CI), stroke volume (SV), heart rate (HR), left ventricular end-diastolic and end-systolic pressure (LVEDP, LVESP), systolic, diastolic and mean aortic pressure (AOsyst, AOdiast, AOmean) were taken in all patients.
STATISTICAL ANALYSIS
Continuous data were described as means and standard deviations. All data were entered into a data table and analysed employing the built-in analysis. Column statistics were used to present results for the baseline parameters and TTE/CT measurements. Intra-individual comparisons of pre-implantation and post-implantation measurements were performed using a paired t-test with the standard error of the mean (SEM). For overall tests, p≤0.05 was considered significant. Statistical analyses were performed with GraphPad Prism version 5 (GraphPad Software, San Diego, CA, USA).
Results
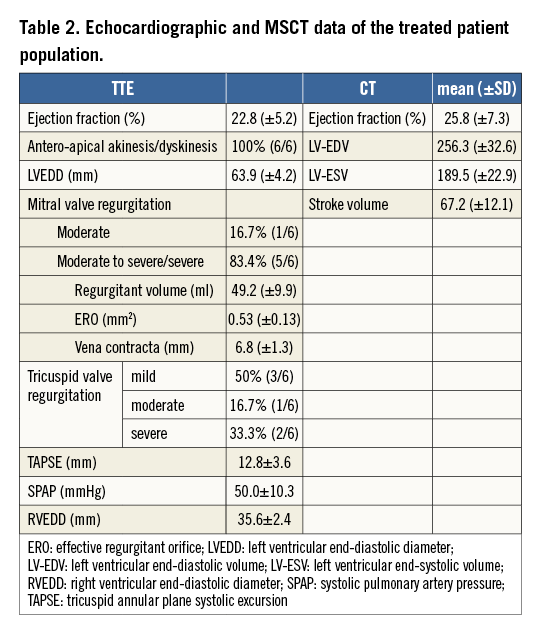
The mean age of the treated patients was 73.8±5.2 and the average logistic EuroSCORE I was 27.9±10%, indicating a rather sick patient population (Table 1). All patients were at least in NYHA Class III or IV and had at least one in-hospital treatment due to worsening in heart failure symptoms within 12 months before Parachute implantation. Moreover, all patients had a prior myocardial infarction due to occlusion of the left anterior descending (LAD) artery, and all patients had an akinesis or dyskinesis of the antero-apical region of the left ventricle. The mean ejection fraction (EF) was 22.3±8.9% measured by TTE. MSCT confirmed enlarged ventricles with impaired LV function (Table 2).
PROCEDURAL DETAILS
Procedural time was 127.5±34.6 min, contrast volume used was 168.5±65 ml, fluoroscopy duration was 58.3±31.1 min (minimum 31 min, maximum 119 min) and mean X-ray exposure was 23,360.6±10,658.1 mGy/cm2. Average Parachute device size was 91.6±5.1 mm (n=4 95 mm, n=2 85 mm, n=5 with a short foot). All procedures were performed through a transseptal approach via a 24 Fr MitraClip guide in combination with a MitraClip procedure. In all patients, the Parachute device was successfully positioned into the intended landing zone with appropriate positioning. Due to the steerable guide of the MitraClip, antegrade and transmitral implantation of the Parachute was carried out without any problems (Figure 1). After Parachute implantation, haemodynamic assessments demonstrated a trend towards improved cardiac output (Online Table 1). There was also some evidence of aggravation of MR after Parachute implantation. This was indicated by an increased colour Doppler signal, as well as by an increase in LA pressures (v-wave before: 35.5±14.9 mmHg; v-wave after: 46.3±14.9 mmHg; p=0.017). Subsequently, all patients were treated with MitraClip implantation, due to severe or moderate to severe mitral valve regurgitation. A total of 12 MitraClips were implanted, and MitraClip implantation was successfully performed with reduction of mitral regurgitation in all patients (grade
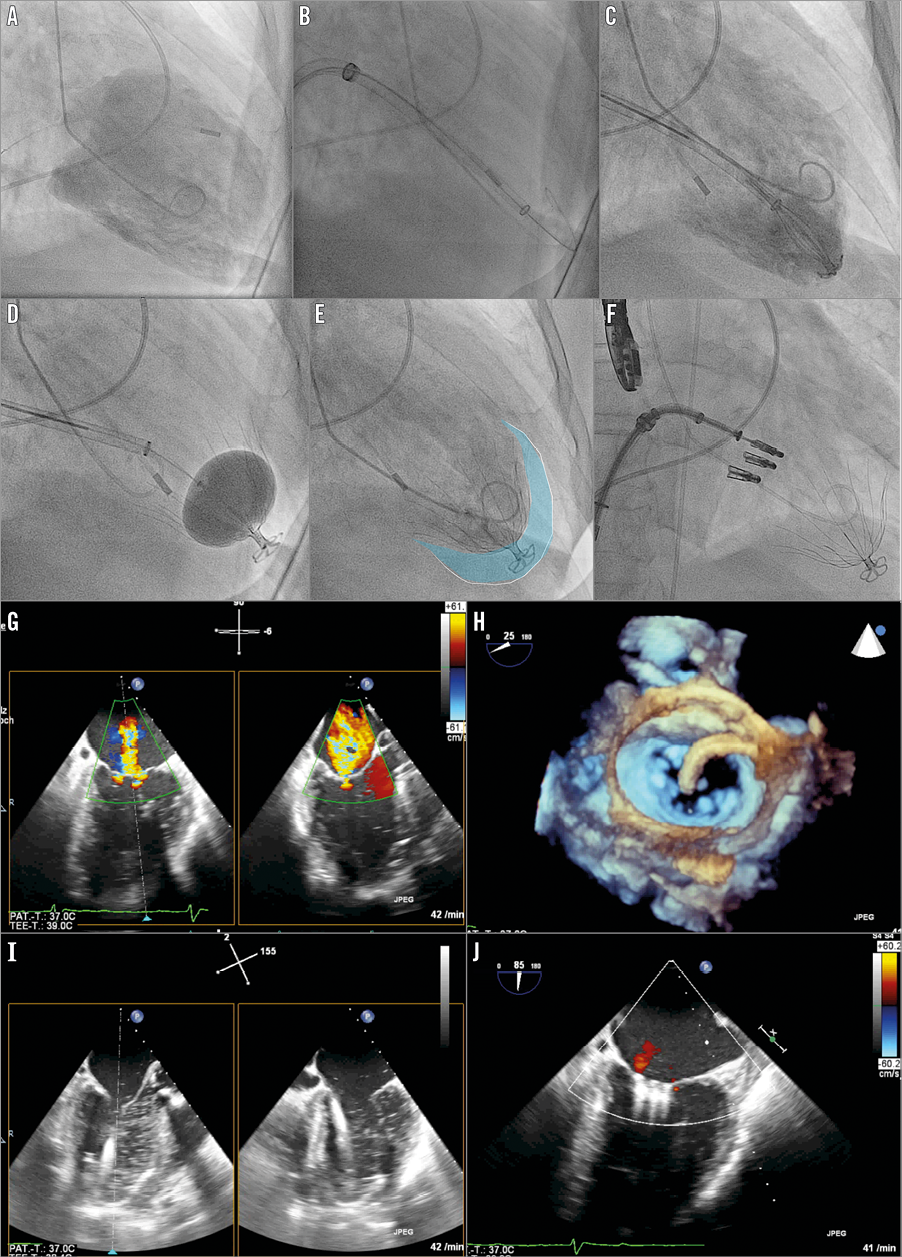
Figure 1. Fluoroscopic images (A-F) and echocardiographic images (G-J). A) Baseline LV angiography. B) Transmitral positioning of Parachute guide. C) Positioning of the Parachute device. D) Ballooning of the Parachute device. E) LV angiography with implanted Parachute device (blue area illustrates the excluded volume). F) MitraClip (n=3) implantation. G) TEE with colour Doppler showing the severe mitral regurgitation before intervention. H) Three-dimensional surgical en face mitral view with the MitraClip guide steering down to the mitral valve. I) Transmitral insertion of the Parachute guide in the left ventricle. J) TEE with colour Doppler showing the final result after MitraClip implantation.
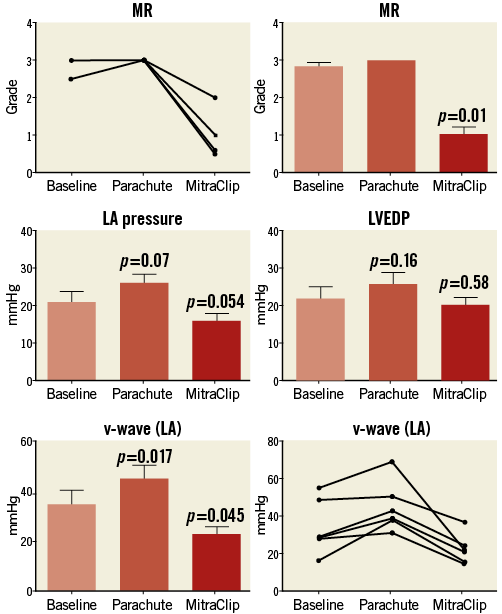
Figure 2. Mitral regurgitation (MR), left atrial (LA) pressure, v-wave and left ventricular end-diastolic pressure (LVEDP) after combined Parachute and MitraClip implantation.
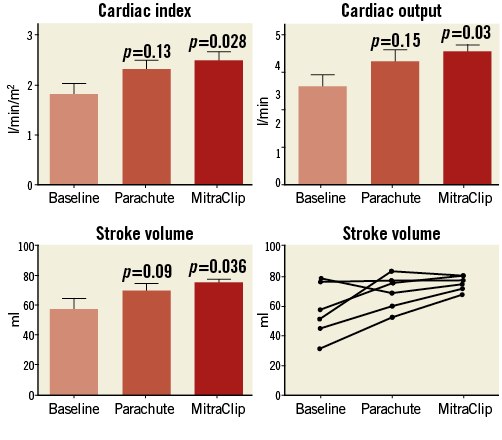
Figure 3. Cardiac index (CI), cardiac output (CO), and stroke volume (SV) after combined Parachute and MitraClip implantation.
SAFETY
There was no incidence of death, stroke, myocardial infarction or respiratory failure periprocedurally and in the immediate postoperative period and within 30 days. There was one case with ventricular arrhythmias during the first 48 hours, well controlled with amiodarone. None of the patients had an ICD shock (80% carrying an ICD/CRT-ICD) during the hospital stay.
EFFICACY
Post-procedure ICU duration was 1.3±0.5 days. All patients achieved MR reduction to 2+ or less at discharge, and patients experienced an improvement in NYHA functional class at discharge. All patients were discharged home after the procedure. At 30 days, three patients were in NYHA functional Class II and three patients were in functional Class III.
POST-PROCEDURAL OBSERVATIONS AND MANAGEMENT
All patients were treated for at least four weeks with clopidogrel and phenprocoumon, followed by phenprocoumon for at least 12 months. There was no bleeding complication during the hospital stay. Patient #6 had to undergo redo MitraClip implantation three months after the index procedure for recurrent severe MR. Two additional clips and a duct occluder (6/4) had to be implanted to repair a tear formation within the anterior mitral leaflet (AML) with a final reduction of MR to trace.
Discussion
The unfavourable natural history in patients with severe ischaemic heart failure warrants still more treatment strategies besides pharmacotherapy. In many instances, myocardial infarction of the left ventricle initiates a process of remodelling, which leads to increasing abnormal wall motion, myocardial thinning and an elongation of the affected region6. The resulting geometrical changes of the infarcted area lead to an increase in wall stress between the infarcted region and normal myocardium13. In this regard, volume reduction with Parachute implantation in patients with an apical left ventricular aneurysm seems to be an appealing and safe concept with potential benefit in functional capacity24,25,28,29,31,38-40. Unpublished (but presented at various international congresses) two-year data after Parachute implantation have shown an improvement in NYHA functional class, quality of life and six-minute walk distance38. In addition, LV volumes were found to be continuously reduced by 15% after one year, and this reduction was sustained over a two-year period with no worsening in heart failure38,40.
However, a retrograde transfemoral approach, the only approved access, is an important limitation for Parachute implantation. Thus, as with transcatheter aortic valve implantation, alternative access paths need to be evaluated since various conditions may preclude a retrograde approach (mechanical or bioprosthetic valves in aortic position, peripheral arterial vascular disease, etc). Finally, one of the more frequent key exclusion criteria for Parachute implantation is severe mitral regurgitation, which is a common finding in patients with congestive heart failure. In fact, mitral regurgitation (3+ to 4+) was found in up to 19% of the enrolled patients in the STICH (Surgical Treatment For Ischaemic Heart Failure) trial41. This population typically represents a patient cohort that could be frequently considered for Parachute implantation after MitraClip implantation (if feasible).
In our database, 49 patients underwent a detailed screening for Parachute implantation. So far, 32 (65%) have qualified anatomically for an implant, and 23 (47%) have undergone a Parachute implantation to date. Of these, six patients received a transseptal implantation (study population+MitraClip) and 17 a retrograde Parachute implantation. Two patients were treated via the retrograde approach through an aortic bioprosthesis (n=1 SAPIEN valve, n=1 PERIMOUNT; Edwards Lifesciences, Irvine, CA, USA) and two other patients received a MitraClip (Abbott Vascular), one as a staged procedure and one through the index procedure, both Parachute implantations being performed via the retrograde approach. Thus, the present report might offer a new solution for non-surgical patients with an LV aneurysm with concomitant severe mitral regurgitation. In addition, an alternative transvenous and transseptal access would spare these patients from an additional large bore arterial access introducer (14-16 Fr for the Parachute device).
The main results of the present study can be summarised as follows. Parachute implantation through a transseptal approach, as alternative access, is feasible and leads to a similar improvement of global cardiac haemodynamics (increase in CO: +36.4%; 0.9±1.2 l/min) compared to the retrograde transfemoral approach (increase in CO: +25.8%; +0.8±0.1 l/min)32. Nevertheless, there was some indication of aggravation of MR (increased colour Doppler signal, higher LA pressures/v-waves) after Parachute implantation, indicating an increased susceptibility for worsening of MR, and thus very likely limiting the beneficial effects with Parachute implantation. In fact, we believe that pre-existing significant MR should always be considered as a contraindication (the more pre-procedural MR was present, the less an increase in CO was found) after Parachute implantation. By concept, the present study was performed with the use of the 24 Fr steerable MitraClip guide catheter for Parachute implantation. The set-up was very similar to a standard MitraClip procedure using the steering options of the MitraClip guide. In addition, the MitraClip guide provided a sufficient back-up, offering fine adjustments to reach an ideal angle to target the intended landing zone for the Parachute device. All six patients were implanted successfully without any technical difficulties. Subsequently, MR was treated by MitraClip implantation without the need for additional vessel punctures, since the MitraClip guide remained in the left atrium (final grade of mitral regurgitation was ≤I+ in five patients and II+ in one patient). On average, two MitraClips were implanted per patient (dependent on the severity of MR), which is in accordance with other published data42-44. After MitraClip implantation, we found an impressive increase in stroke volume (+44.3%; p=0.03) and cardiac output (+44.5%; p=0.03), which was even more pronounced compared to Parachute implantation alone32.
In summary, the study demonstrates that the transseptal approach for Parachute implantation can easily be combined with a MitraClip procedure, if the transseptal access is carefully chosen as being appropriate for MitraClip implantation. In fact, all six Parachute implantations were successfully performed through an antegrade transseptal approach. Finally, we believe that the combined implantation of a Parachute and MitraClip(s) may provide a synergistic benefit with regard to global cardiac haemodynamics (Figure 4).
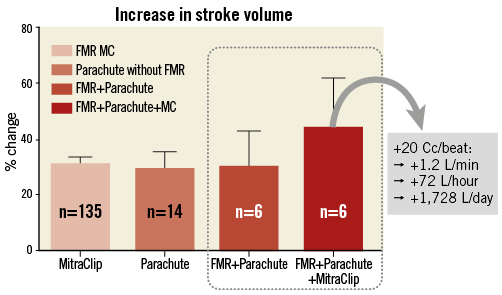
Figure 4. Percent increase in stroke volume (SV) with MitraClip implantation alone (n=135 patients with functional mitral regurgitation), with Parachute implantation alone (n=14 patients with left ventricular apical aneurysm and without significant mitral regurgitation, i.e. MR Limitations This is a small study of six consecutively treated patients. We were intrigued to try this approach despite an adequate arterial femoral access in most patients. Furthermore, it needs to be mentioned that the retrograde implantation of a Parachute device (standard approach) is rather straightforward to perform and is currently the only approved methodology (carrying CE mark only). Nevertheless, implantation of the device through a transvenous and transseptal approach yielded results comparable to the retrogradely implanted patients. However, the small sample size does not preclude an additional risk of iatrogenic injuries to the interatrial septum, as observed in patient #6. Thus, re-evaluation of this additional access technique for Parachute implantation might be of interest in the future. Conclusion The current study demonstrates the feasibility of the transseptal access for Parachute implantation. Moreover, it provides additional evidence of efficacy to the Parachute studies, which have previously demonstrated the ventricular partitioning device to be safe and of potential benefit in patients with heart failure. Evidently, device efficacy and safety with the transseptal and transmitral approach needs to be demonstrated in a larger study group. Impact on daily practice Transvenous and transseptal Parachute implantation in conjunction with MitraClip therapy in patients with left ventricular apical aneurysms and severe mitral regurgitation might be an interesting hybrid concept in the future, since this approach might be applicable to many patients (roughly 20% of patients with LVAA display more than moderate MR according to the STICH trial21). The present study demonstrates the feasibility in only six patients, thus more data regarding safety and efficacy need to be documented. Nevertheless, the combined approach is currently considered as a primary therapy at our centre for high-risk surgical candidates (not eligible for LVAD or mitral surgery), because we consider severe MR as a true contraindication for standalone PI in LVAA. Conflict of interest statement U. Schäfer is a consultant to CardioKinetix and has received speaker honoraria and travel support. The other authors have no conflicts of interest to declare. Supplementary data Online Figure 1. Preparation of the MitraClip guide with a regular 10 Fr sheath inside the haemostatic valve to prevent air entrapment during guidewire manipulation. The side port of the MitraClip guide is connected to a flush-line and a 50 cc syringe to remove air and to flush the system with positive pressure during insertion of the Parachute guide catheter inside the MitraClip guide.