Abstract
ICD patients with narrow QRS, CRT non-responders, and functional MR patients all have one mechanistic failure mode that is left untreated – the scar left behind following an MI. ICDs, CRTs, and MitraClip implantation are all well-proven therapies, but the Parachute device may address the mechanistic void that remains after each of these therapies has been used and may further improve patients’ outcomes. A pooled analysis of 134 subjects was conducted using the first three clinical trials which included subjects with symptomatic ischaemic HF with LV wall motion abnormalities secondary to MI, and an LV ejection fraction less than 40%. The two-year cumulative mortality rate was 12.9%, with 8.7% in the first year and an increment of 4.2% in the second, which is a 53% reduction as compared to the first year. There is a significant proportion of patients with ischaemic heart failure being excluded from cardiac rhythm management (CRT, etc.), leaving a large treatment gap until mechanical support devices (LVAD) or heart transplantation in progressive heart failure are indicated. Along with other heart failure devices, Parachute may be a useful treatment modality, addressing a mechanistic void in the treatment of this disease. Current data support improvements in haemodynamics, functional capacity, six-minute walk distance, quality of life and a promising decline in mortality two years after Parachute implantation.
Target patients
A common thread exists among patients with narrow QRS usually treated with an implantable cardioverter-defibrillator (ICD), cardiac resynchronisation therapy (CRT) non-responders, and those with functional mitral regurgitation (FMR). All have one mechanistic failure mode which is left untreated, namely the subsequent scar left behind following a myocardial infarction (MI) (Figure 1A). The myocardial scar causes a non-synchronous LV contraction and is the precursor to LV remodelling causing increased wall strain and reduced cardiac output which: 1) has been shown to cause sudden cardiac death (SCD), 2) has been shown to be a significant factor in non-responder CRT patients, and 3) is a primary cause of FMR. ICDs, CRTs, and MitraClip® (Abbott Vascular, Santa Clara, CA, USA) implantation are all well-proven therapies, but the Parachute® system (CardioKinetix, Menlo Park, CA, USA) which is designed to treat non-normal (akinetic or dyskinetic) LV apical wall motion secondary to an anterior MI may address the mechanistic void that remains after each of these therapies has been used and may further improve patients’ outcomes. As it stands today, approximately 30-40% of patients have been shown to be non-responders to CRT1,2 and, even worse, up to 22% have been shown to be adverse responders with increased rates of mortality, hospitalisation for heart failure (HF) or heart transplantation2. Predictors of non-response are ischaemic cardiomyopathy, the myocardial scar, right bundle branch blockade (RBBB), absence of dyssynchrony, and poor lead placement or anatomy1. In addition, the commonly observed prevalence of atrial fibrillation in patients with heart failure (up to 25%, 50% in Class IV) limits the efficacy of CRT3. Last but not least, no clinical trial has shown clinical benefit without QRS prolongation >120 ms4,5. Hence, there is a huge proportion of patients with systolic HF which might be addressable with other therapeutic devices.
Study device
The Parachute system includes the device, a delivery system with a balloon that facilitates expansion of the device, and a pre-shaped delivery catheter and dilator. The Parachute device comprises a self-expanding nitinol frame, an ePTFE impermeable membrane, and an atraumatic polymer foot (Figure 1B). It comes in eight sizes (65, 75, 85 and 95 mm diameter, each offered in two “foot” heights, short and standard). The distal atraumatic foot is radiopaque and provides a contact point with the LV apical wall. The contact point is selected in order to orient the device with a vector towards the LV outflow tract. The device is mainly delivered under fluoroscopic guidance via a transfemoral retrograde transaortic approach (Moving image 1, Figure 2).
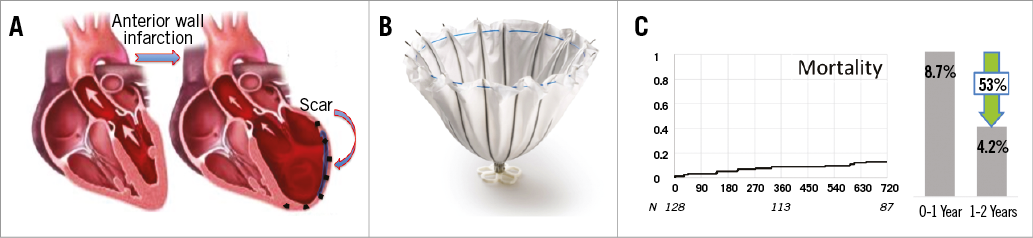
Figure 1. Pathomechanism, device and outcome. A) Anterior wall infarction leading to antero-apical aneurysm formation; B) the Parachute implant; C) pooled mortality data from the Parachute trials I, II, III.
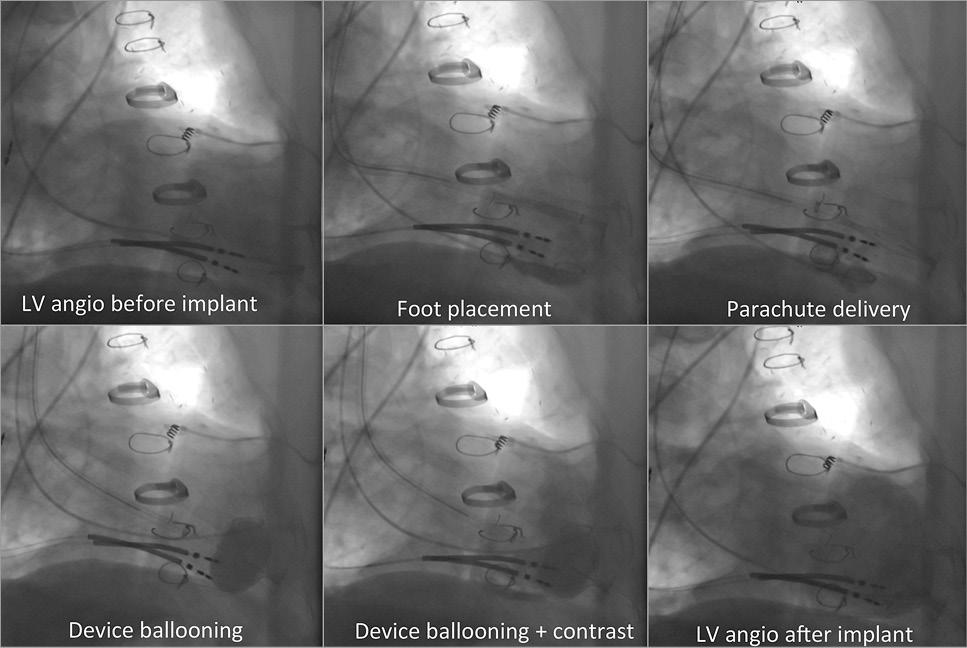
Figure 2. Step-by-step implantation guide.
Parachute results
The first Parachute device was implanted in 2005. Since then, there have been three clinical trials that have completed enrolment and followed the patients for a minimum of two years. A pooled analysis of 134 subjects was conducted using the first three clinical trials that included subjects with symptomatic ischaemic HF with LV wall motion abnormalities secondary to MI, and an LV ejection fraction less than 40%. The end-systolic volume index remained reduced at two years (77.3±21.1 mL/m2 compared to a baseline value of 89.1±21.4 mL/m2, p<0.0001), and a reduction in the wall motion severity index was observed (2.5±0.3 versus 2.2±0.2, p<0.0001). The mean NYHA class of subjects at baseline was 2.6±0.5. This was significantly reduced after two years (p<0.001) to a mean NYHA class of 2.2±0.8, reflecting functional improvement. The two-year cumulative mortality rate was 12.9%, with 8.7% in the first year and an increment of 4.2% in the second, which is a 53% reduction as compared to the first year (Figure 1C). This promising plateauing effect does differ from that commonly seen in other heart failure trials where the cumulative mortality rate ranges to more than 20% and is linear across years one and two6,7.
It is common for patients with ischaemic cardiomyopathy and apical wall motion abnormality also to have FMR. A pilot study from the University Heart Center Hamburg, Germany, showed improvement in patients suffering from FMR after receiving both the Parachute and MitraClip8. Parachute implantation (PI) was performed via a transseptally placed MitraClip guide in six consecutive patients. Immediately after PI, MR was treated by MCT. Invasive right and left heart haemodynamics were measured before and after PI and MCT, respectively. Procedural success was 100%. PI and MCT induced a significant increase in stroke volume (SV: +44.3%, p=0.03) and cardiac output (CO: +44.5%; p=0.03).
Parachute mechanism of action
The trigger of ischaemic HF is the non-synchronous scar with remodelling as the compensatory response to the deleterious systolic eccentric LV wall motion. The mechanistic theory of the Parachute is: 1) to reduce volume thereby reducing wall stress in the upper chamber of the LV, and 2) to synchronise wall motion during systolic contraction by replacing the eccentric wall motion in the apical region with a more compliant device. The benefits of reducing wall stress and synchronising apical wall motion throughout the cardiac cycle allow improved cardiac output and reduced filling pressure.
Regarding the first mechanism of action, volume reduction, the ESV reduced by the Parachute was sustained at one year (range between 30 mL and 40 mL). A recent meta-analysis using mortality data from 30 trials with a median follow-up of 17 months highlighted the critical role of LV end-systolic and end-diastolic volume reduction for the success of pharmacological and mechanical approaches to HF9. A curve from this publication correlates the amount of end-systolic volume (ESV) reduction with the predicted probability of showing a reduction in mortality. The ESV reduction shown by the Parachute correlates to an approximately 90% probability of showing a reduction of mortality.
Regarding the second mechanism of action, synchronised wall motion, the frame of the Parachute is actively engaged into active mid LV contractility. This results in synchronised contraction throughout the entire LV, including the apex. The collective mechanistic actions by the Parachute may yield an acute improvement in cardiac function. Following Parachute implantation, a significant increase in stroke volume (+25.4%, p=0.0005), stroke volume index (+26.5%, p=0.0005), cardiac output (+25.8%, p<0.0001) and cardiac index (+25.9%, p<0.0001) has also been described10.
Limitations
As per the current labelling, oral anticoagulation for at least one year is mandatory, precluding patients with contraindications to warfarin therapy. In consideration of future VT ablation, the Parachute may limit subsequent endocardial ablation of VT if that should be targeted in the lower 30-40 mm of the left ventricle. Last but not least, there is some concern for left ventricular assist device (LVAD) implantation after PI with worsening in heart failure, although several case studies have demonstrated the feasibility of Parachute removal and LVAD implantation.
Conclusions
Up until now, a significant proportion of patients with ischaemic heart failure have been excluded from cardiac rhythm management (CRT, etc.), leaving a large treatment gap until mechanical support devices (LVAD) or heart transplantation in progressive heart failure are indicated. Along with other heart failure devices, the Parachute system may be a useful treatment modality, addressing a mechanistic void in the treatment of this disease. Current data support improvements in haemodynamics, functional capacity, six-minute walk distance, quality of life and a promising decline in mortality two years after Parachute implantation. A randomised controlled trial (PARACHUTE IV, currently underway) will elucidate whether Parachute can reduce mortality and re-hospitalisation for heart failure episodes compared to optimal medical therapy, thereby possibly influencing current treatment strategies.
Conflict of interest statement
U. Schäfer is a paid consultant and proctor for CardioKinetix. In addition, he has received speaker’s honoraria and travel support from CardioKinetix.
Supplementary data
Moving image 1. Angiographic step by step description of a Parachute implantation.
Supplementary data
To read the full content of this article, please download the PDF.
Angiographic step by step description of a Parachute implantation.

