
Introduction
Left ventricular aneurysms (LVAs) are found in 10 to 30% of patients suffering from anterior myocardial infarction1. Traditionally, scar reduction has required the use of invasive surgical techniques2. The Surgical Treatment for Ischemic Heart Failure (STICH) trial is a classic trial comparing coronary artery bypass grafting (CABG) alone with a combined procedure of CABG and surgical ventricular reconstruction3. Currently, catheter-based procedures for direct modification of the left ventricle are represented by the Parachute device (CardioKinetix Inc., Menlo Park, CA, USA) and the Revivent TC™ device (BioVentrix Inc., San Ramon, CA, USA)4,5. We report our single-centre experience and outcomes of 26 patients undergoing the Revivent “Less Invasive Ventricular Enhancement” procedure, which requires no sternotomy, no ventriculotomy, and no extracorporeal or circulatory support.
Methods
THE REVIVENT TC PROCEDURE
The Revivent procedure involves plication and exclusion of the left ventricular (LV) scar using paired micro-anchors. One anchor is implanted surgically into the scarred area of the LV epicardium whilst the other is introduced percutaneously into the right side of the interventricular septum. The most apical aspect of the LV is then excluded. The detailed steps of the procedure are illustrated in Supplementary Figure 1 and Supplementary Appendix 1.
STUDY SUBJECTS, INCLUSION AND EXCLUSION CRITERIA
This is a prospective study of patients who underwent the Revivent TC procedure from January 2017 to January 2019 at a single centre. It has received approval from the Institutional Ethics Committee. All patients provided written informed consent prior to enrolment into this study. The inclusion and exclusion criteria are shown in Supplementary Appendix 1.
Results
From January 2017 to January 2019, 26 patients underwent a Revivent TC procedure (Supplementary Table 1). In the follow-up period, two patients suffered from major adverse cardiac events (MACE), which corresponds to a primary event rate of 7.7%. One was re-hospitalised three times for recurrent heart failure; the other patient died on day 56 from multi-organ system failure.
LEFT VENTRICLE AND LV ANEURYSM DIMENSIONS
As shown in Figure 1A, LV end-diastolic diameters (LVEDD) obtained from the different views were significantly reduced (LVEDD-AP, by echocardiography, 63.6±7.1 to 55.2±7.7 mm, p<0.001), (LVEDD-MA, by cardiac magnetic resonance [CMR], 83.8±10.3 to 73.6±9.9 mm, p<0.001), (LVEDD-LS, by CMR, 61.6±8.7 to 58.4±8.1 mm, p=0.021). LV end-systolic volume (LVESV) (139.5±49.6 to 107.8±43.8 ml), LVSEV index (84.8±25.7 to 65.6±24.4 ml/m²), LV end-diastolic volume (LVEDV) (189.8±57.2 to 158.4±55.0 ml) and LVEDV index (107.8±33.2 to 90.5±31.8 ml/m²) were all significantly reduced (p<0.001). The LV aneurysm end-diastolic diameters measured between the top and bottom of the aneurysm in the two-chamber (LVAEDD2Ch 43.8±12.8 to 32.1±12.4 mm, p<0.001) or four-chamber view (LVAEDD4Ch 40.1±8.8 to 32.2±9.2 mm, p<0.001) and between the neck and the tip of the aneurysm in the four-chamber view (LVAEDD4Ch, neck 41.7±10.6 to 30.1±9.0 mm, p<0.001) by CMR were also significantly reduced.
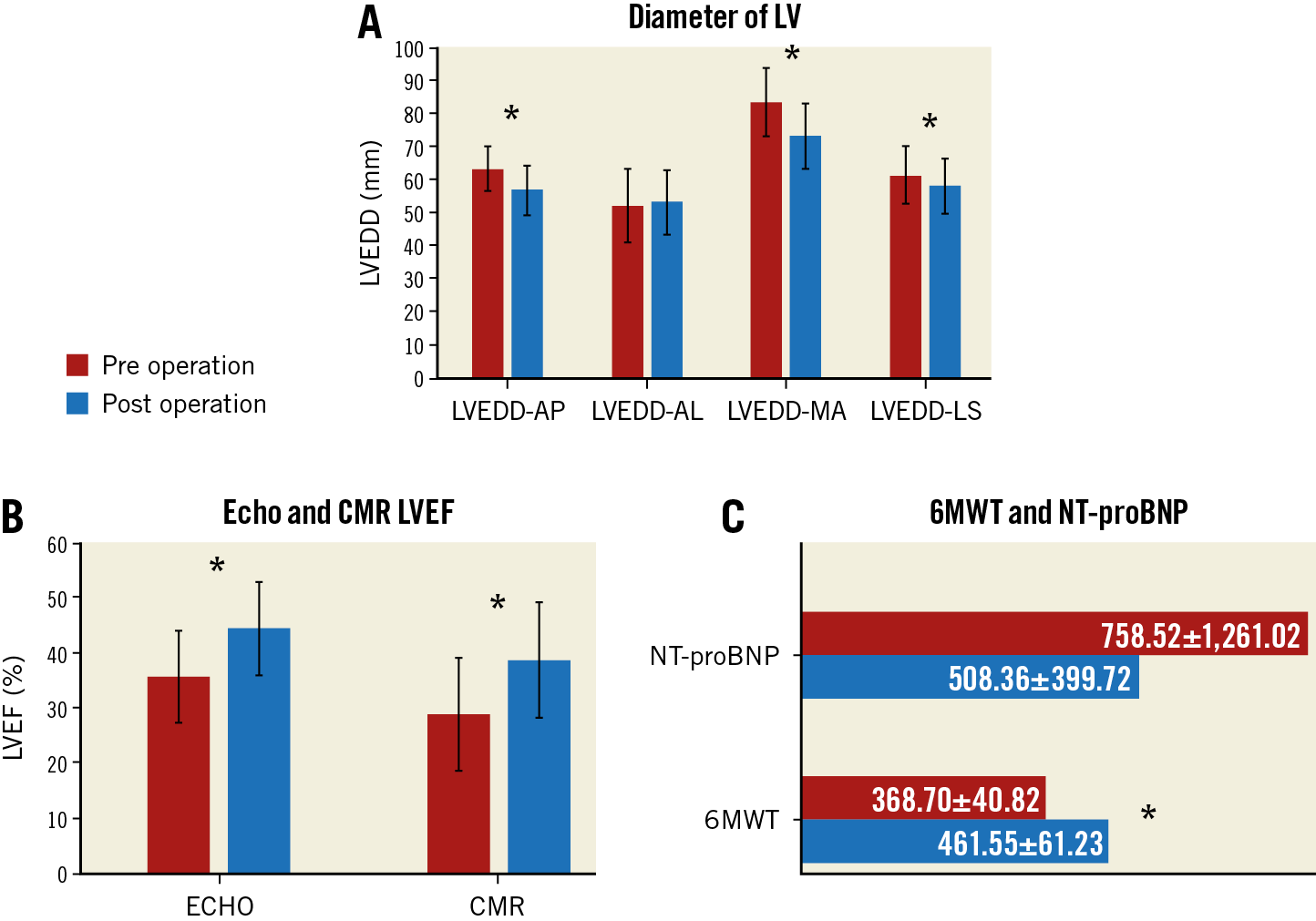
Figure 1. LV end-diastolic diameter and cardiac function markers pre and post Revivent TC operation. A) LVEDD in different views by echocardiography and CMR. B) LVEF by echocardiography and CMR. C) NT-proBNP and six-minute walking test (6MWT). LVEDD-AL: LV end-diastolic diameter (LVEDD) measured between the anterior and lateral walls in the four-chamber view by CMR. LVEDD-AP: LVEDD measured between the anterior and posterior dimension in the long-axis view on echocardiography; LVEDD-MA: LVEDD measured between the mitral valve and the apex in the four-chamber view by CMR; LVEDD-LS: distance between the lateral wall and the septum on the short-axis view at the papillary muscle level by CMR.
CARDIAC FUNCTION
A series of echocardiographic and CMR examinations was performed at different time points to determine the progression in cardiac function. Left ventricular ejection fraction (LVEF), determined by echocardiography and CMR imaging (Figure 1B), was significantly improved (echocardiography: 35.6±8.8% vs 45.9±9.8%, p<0.001; CMR: 28.9±8.3% vs 38.6±10.5%, p<0.001). The six-minute walk test distance was significantly longer (368.8±40.0 to 461.5±61.2 m, p<0.001). There was no change in NT-proBNP (758.6±1,261.1 to 508.4±399.1 pg/ml, p=0.916) (Figure 1C), but New York Heart Association (NYHA) heart failure class was improved at nine months (2.7±0.6 to 1.7±0.7, p<0.001).
Discussion
This study is the largest prospective study published thus far for patients undergoing left ventricular enhancement using the Revivent TC procedure, which is performed on a beating heart without sternotomy, ventriculotomy, or cardiopulmonary bypass. The main findings are that it effectively reduced LVESV, and improved LVEF, with a good safety profile, as well as acceptable complication rates and mortality endpoints. We demonstrate that this procedure can be performed safely by cardiologists with appropriate training. The novelty is the use of CMR for accurate structural and functional characterisation of cardiac function.
All patients successfully received the implantation of the micro-anchors, with a procedural success rate of 100%, and with no device implantation failure. Only two patients suffered from MACE in the follow-up of nine months. With the accumulation of experience, operative time improved from 360 min for the first case to 240 min for the last case, demonstrating the presence of an acceptable learning curve effect for experienced interventional cardiologists and cardiac surgeons.
Limitations
There are several limitations of our study. Firstly, this is an observational study and was not designed to compare outcomes with patients undergoing surgical treatment or receiving only medical therapy. Another limitation is that it is only a single-centre study involving a small number of patients with relatively short follow-up.
Conclusion
The Revivent TC procedure was able to provide significant benefits in terms of left ventricular volume, ejection fraction, six-minute walk test and NYHA heart failure class nine months after the operation. Its long-term efficacy and safety should be confirmed by larger prospective studies with longer follow-up durations.
|
Impact on daily practice The Revivent TC procedure is a minimally invasive hybrid operation. It is safe, effective, performed on a beating heart without ventriculotomy or cardiopulmonary bypass, and results in a significantly smaller LV, higher EF and improved function with few adverse events. |
Funding
The study was funded by research grants from the Xiamen Health Commission (3502Z20179049), Xiamen Science and Technology Bureau (3502Z20151041) and Fujian province (2016-CXB-13).
Conflict of interest statement
L. Annest is an employee of the BioVentrix company. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

